Stainless steel is a family of corrosion resistant steels containing chromium in which the chromium forms a passive film of chromium oxide (Cr2O3) when exposed to oxygen [1]. This phenomenon is called passivation and is seen in other metals, such as aluminium and titanium.
The film layer is impervious to water and air and quickly reforms when the surface is scratched. This protects the metal beneath – preventing further surface corrosion. Since the layer only forms in the presence of oxygen, corrosion-resistance can be adversely affected if the component is used in a non-oxygenated environment e.g. underwater bolts on a platform support structure.
Such passivation only occurs if the proportion of chromium is high enough and is normally achieved with addition of at least 13% (by weight) chromium. Progressively higher levels of corrosion resistance and strength is achieved by the addition of other alloying elements – each offering specific attributes in respect of strength and corrosion resistance.
Classification issues
The need to classify stainless steel has led to a fundamental problem of which method to use. Probably the best known system derives from of the Society of Automobile Engineers (SAE) e.g. 316 Cr/Ni/Mo 17/12/2. This is interpreted as stainless steel containing the proportions of 17% chromium, 12% nickel, and 2% molybdenum.
However, the waters are somewhat muddied by a variety of international and country-based systems that include EN (European Norm); and UNS (Unified Numbering System). For example, SAE 304 Cr/Ni 18/10 stainless steel is EN 1.4301 which is UNS S30400.
Stainless steels may also be graded into five basic families or phases determined by their crystalline structure: the stable phases austenitic or ferritic; a duplex mix of the two; the martensitic phase created when some steels are quenched from a high temperature; and precipitation-hardenable.
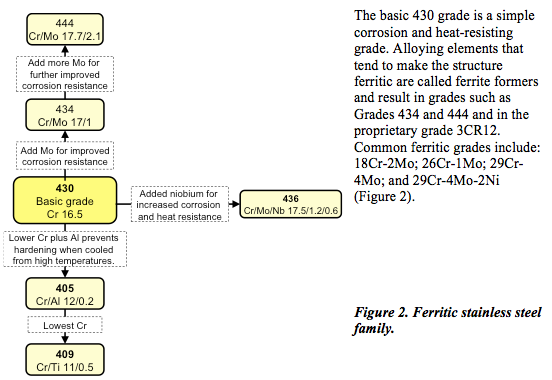
Ferritic stainless steel
In ferritic stainless steel, the iron and chromium atoms are arranged in what is termed a body-centred cubic structure in which the atoms are arranged on the corners of the cube and one in the centre (Figure 1). As well as being ferro-magnetic, ferritic stainless steel exhibits very high stress corrosion cracking resistance.

Ferritic stainless steels are plain chromium (10.5 to 18%) grades characterized by moderate corrosion resistance and poor fabrication properties. These characteristics may be improved with the addition of molybdenum; some, aluminium or titanium.
Austenitic stainless steel
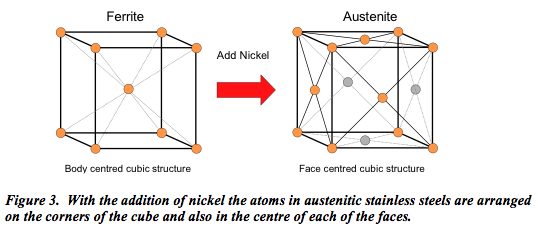
With the addition of nickel, the properties change dramatically. As shown (Figure 3) the atoms are re-arranged so that they occur on the corners of the cube and also in the centre of each of the faces. In this manner it becomes what is termed austenitic stainless steel.
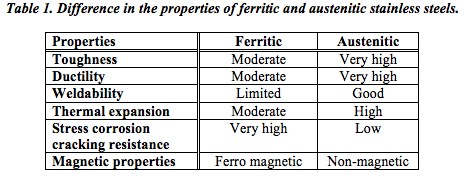
It can thus be seen from Table 1 that unless you are specifically looking for a ferro-magnetic material, austenitic stainless steel would be the most obvious choice. Indeed this is borne out by the fact that austenitic stainless steels account for about 70% or more of all stainless steel used worldwide – with ferritic stainless steels making up about 25%. The other families each represent less than 1% of the total market.
Austenitic stainless steels are designated by numbers in the 200 and 300 series.
Series 300
The relationship between the 300 austenitic grades is shown in Figures 4.
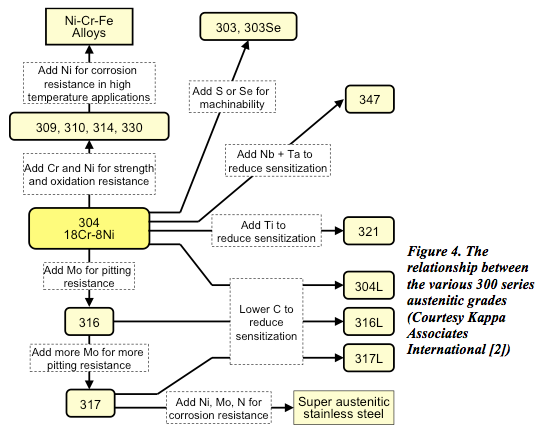
The basic grade 304 contains about 18% chromium and 8% nickel (often referred to as 18/8) and range through to the high alloy or ‘super austenitics’ such as 904L and 6% molybdenum grades.
Additional elements can be added such as molybdenum, titanium or copper, to modify or improve their properties, making them suitable for many critical applications involving high temperature as well as corrosion resistance. This group of steels is also suitable for cryogenic applications because the effect of the nickel content in making the steel austenitic avoids the problems of brittleness at low temperatures, which is a characteristic of other types of steel.
Generally, the 300 grade alloys are subject to crevice and pitting corrosion.
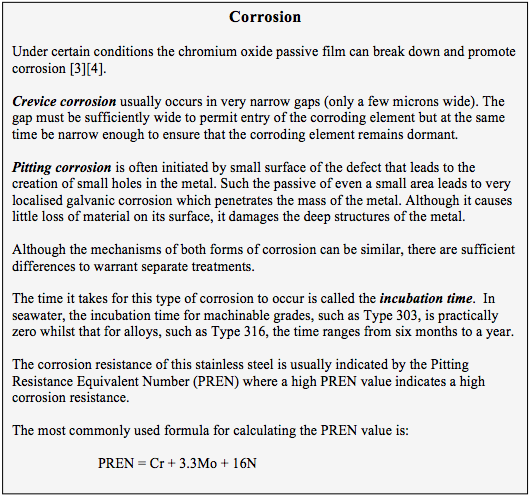
Low-carbon versions, (indicated by the letter suffix L) include 304L, 316L and 317L, in which the carbon content of the alloy is below 0.03%. This reduces the effect of ‘sensitisation’ in which chromium carbides precipitate at the grain boundaries due to the high temperatures involved in welding. The relatively high nickel content also inhibits the brittleness exhibited by ferritic materials at low temperatures and thus makes austenitic steels suitable for cryogenic applications.
200 series
We have seen earlier how the addition of nickel is used in the creation of the classic chrome-nickel 300 series austenitic stainless steel.
The reduced nickel content of the 200 series chrome-manganese grades makes them significantly cheaper. However, depending on their chemistry, they also offer good formability (ductility) and/or strength. Indeed, certain grades (201, 202 and 205 series) even offer about 30% higher yield strength than the classic 304-series chrome-nickel grade – allowing designers to cut weight (Table C.2).
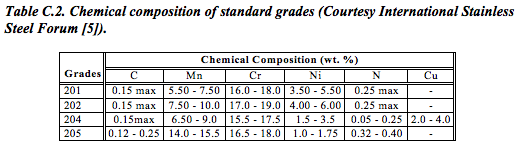
Reducing nickel, on the other hand, reduces the maximum chromium content possible in the alloy. Less chromium means less corrosion resistance and a consequent narrowing of the range of applications for which the material is suitable.
A word of warning comes from the International Stainless Steel Forum (ISSF). Continuous pressure to cut costs, especially from the Asian market, has resulted in the development of austenitic grades ever lower in nickel and chromium, often not covered by international codes or specifications. In fact, numerous chrome-manganese grades are company-specific and identified simply by a title given to them by the producer.
Duplex stainless steels
Duplex stainless steels [6] are a mixture of austenite and ferrite microstructures that combine some of the features of each class:
- resistance to stress corrosion cracking – but inferior to ferritic steel;
- superior toughness to ferritic steel – but inferior to austenitic steel;
- roughly twice the strength of austenitic steel;
- superior resistance to pitting, crevice corrosion and stress corrosion cracking;
- high resistance to chloride ions attack; and
- high weldability.
These features are achieved by adding less nickel than would be necessary for making a fully austenitic stainless steel. Thus, Grade 2304 comprises 23% chromium and 4% nickel whilst Grade 2205 comprises 22% chromium and 5% nickel – with both grades containing further minor alloying additions.
On the negative side, austenitic-ferritic duplex stainless steels are only usable between temperature limits of about -50°C and 300°C – outside which they suffer reduced toughness.
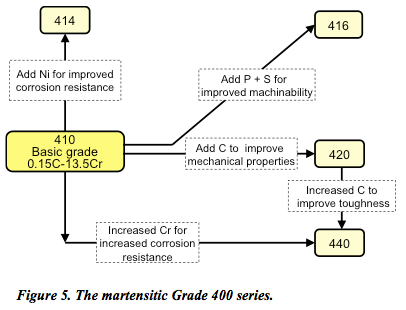
Martensitic stainless steel
Named after the German metallurgist, Adolf Martens, the martensitic Grade 400 series (Figure 5) are low carbon (0.1–1%), low nickel (less than 2%) steels containing chromium (12 to 14%) and molybdenum (0.2–1%).
Stainless steels hardened by transformation to martensite are tempered to give the desired engineering properties. At high temperatures they have an austenitic structure that is transformed into martensitic structure upon cooling to room temperature. Unfortunately, this tempering can influence corrosion susceptibility. For example, corrosion susceptibility of type 420 stainless steel is at its maximum when the alloy is tempered at temperatures in the range of 450° to 600°C. So, aalthough not as corrosion-resistant as the 200 and 300 classes, martensitic stainless steels are magnetic, extremely strong (if not a little brittle), highly machinable, and can be hardened by heat treatment.
Martensitic stainless steels are subject to both uniform and non-uniform attack in seawater. And the incubation time for non-uniform attack in even weak chlorides is often only a few hours or days.
Precipitation-hardening martensitic stainless steels
These chromium- and nickel-containing steels can be precipitation hardened to develop very high tensile strengths. Precipitation-hardening stainless steels are usually designated by a trade name rather than by their AISI 600 series designations.
The most common grade in this group is ‘17-4 PH’, also known as Grade 630, with a composition of 17% chromium, 4% nickel, 4% copper and 0.3% niobium. The main advantage of these steels is that they can be supplied in the ‘solution treated’ condition – in which state the steel is just machinable. Following machining, forming, etc. the steel can be hardened by a single, fairly low temperature ‘ageing’ heat treatment that causes no distortion of the component.
Precipitation hardening generally results in a slight increase in corrosion susceptibility and an increased susceptibility to hydrogen embrittlement.
By: Mick Crabtree
References
1. T. Sourmail and H. K. D. H. Bhadeshia, ‘Stainless Steels’, University of Cambridge.
2. Nabil Al-Khirdaji, ‘Stainless Steel Family’, Kappa Associates International.
3. ‘Stainless Steel and Corrosion’, ArcelorMittal, Stainless Europe.
4. A.U. Malik, M. Kutty, Nadeem Ahmad Siddiqi, Ismaeel N. Andijani, and Shahreer Ahmad, ‘Corrosion Studies on SS 316 L in low pH high Chloride product water medium’, 1990.
5. ‘The Stainless Steel Family’, International Stainless Steel Forum.
6. API Technical Report 938-C: ‘Use of Duplex Stainless Steels in the Oil Refining Industry’.

Excellent article on stainless.
would like to include my perosnla emai
muru.ayyappan@yahoo.com
we are suspecting stress corrosion cracking problème of a heat exchanger tubes. In the exchanger data sheet they mensinned the tube meterial which is Stainless Steel type A179. WHAT IS THAT MEANS? and to what family it belongs?
regards
A-179 is not a stainless steel. It is a low carbon seamless heat exchanger tube.
Very Interesting Article!!!
Interesting and helpful article
Thank you Doug! It DEFINITELY feels Will has NOT done his homework on Exactly what Cannabis is, or DOES! Seems to be a machine for the Bad guys, the Corrupt ones!!!
Hello there, I ran across your web site by way of Yahoo simultaneously because trying to find a equivalent subject, your website showed up, it seems like fantastic. We’ve bookmarked this within my yahoo social bookmarks.
Hello. thank for the post.
Of course, there are many who start to european union 2004 follow.
For Mr Roy a business plan? This is why small business consultants are very much
successful with their objective.
My brother suggested I might like this web site. He used to be
totally right. This submit truly made my day. You can not believe simply how so much time
I had spent for this information! Thank you!
It’s not mmy first time to pay a quick visit this web page, i
am browsing this web site dailly and obtain fastidious
informatikn from here all the time.
my website; thesemid century
Good day! I know this is kind of off topic but I was wondering which blog
platform are you using for this site? I’m getting tired of WordPress because
I’ve had issues with hackers and I’m looking at options for another platform.
I would be great if you could point me in the direction of a good platform.
Feel free to surf to my web site :: homes for rent clearwater fl
Really? This may appear critical, but nonetheless I strongly advise you to reconsider what you just stated. This is totally messed up. Email me at vengasbong@gmail.com
Do you have a spam issue on this website; I also am a blogger,
and I was wondering your situation; many of us have developed some nice practices and we
are looking to trade strategies with others,
why not shoot me an e-mail if interested.
Pretty! This was a really wonderful article. Thank you for providing this info.
My sige outdoor pool loungers
Hi there! Do you use Twitter? I’d like to follow you if
that would be ok. I’m definitely enjoying your blog and
look forward to new updates.
What i do not realize is in fact how you are no longer actually
much more smartly-liked than you might be now. You are very intelligent.
You recognize therefore significantly when it comes to this matter,
produced me personally believe it from so many varied angles.
Its like men and women aren’t involved unless it is something to do with Woman gaga!
Your own stuffs nice. All the time care for it
up!
Feel free to surf to my blog: psn code Generator download – freepsncode.page.tl
–
Well let Pinnacle Self Defense help you gather a self defense plan that will make you without a doubt more secure, confident and ready
for most any attack. A beginner should learn as
much as possible so you do not make a uniformed decision.
Also, they learn to deal with others and make new friends.
My blog post ju jutsu
Thanks for sharing your thoughts on call of duty
moon hack tool. Regards
Have you ever considered writing an ebook or guest authoring on other sites?
I have a blog based on the same information you discuss and
would really like to have you share some stories/information. I know my viewers would value your work.
If you are even remotely interested, feel free to shoot me an email.
What’s up it’s me, I am also visiting this website on a regular
basis, this sit is genuinely good and the visitors aree really sharing pleasant thoughts.
Feel free too visit my web site; weigh loss [Roberto]
Hi, I check your neww stuff on a regular basis.
Your humoristic style is awesome, keep doing what you’re
doing!
My homepage Weight Loss Percentage Calculator
Are you taking care of some unique landscaping ideas. In conclusion,
few diy projects offer you more tangible rewards when compared to a vegetable garden, which is not only attractive to look
at, but gives you something to eat as well. For relationships we ask one to look for things
in “two’s”. Once you’ve chose a design, you’ll be able to start picking out the types of plants you want to use,
and also any hardscape elements for example walkways, patios or decks.
A diy project does not have access to to take a lot of time or cash in order to
create great benefit.
Have you ever thought about creating an ebook or guest authoring on other blogs?
I have a blog based upon on the same ideas you discuss and
would love to have you share some stories/information. I know my audience would appreciate your work.
If you are even remotely interested, feel free to send me an email.
Nice post. I used to be checking continuously this weblog
and I’m impressed! Extremely helpful info particularly the last section 🙂 I take care
of such info a lot. I used to be looking for this certain information for a long time.
Thanks and best of luck.
Suffice it to say, these cutting edge devices that take no prisoners.
Unlike about to malls and visiting countless stores before you decide to find a bargain, you can visit several virtual stores within minutes on the World wide
web. Xbox 360 Wireless Controller: This award-winning, high-performance wireless controller features the Xbox Guide Button for quick, in-game access to friends and
music.
Roofers will hire this dimension dumpster rental in wabash IN to throw outdated
shingles and in addition boards away.
That is a great tip especially to those fresh to the blogosphere. Simple but very precise information… Thanks for sharing this one. A must read post!
[…] need. If you’re looking for cheap cover, then you should certainly avoid buying such small business insurance toronto. Get professional advice if you’re uncertain whether or not you need a particular type of […]
You’re so interesting! I do not think I have read through a
single thing like that before. So wonderful to discover another person with a few unique thoughts on this subject.
Really.. thank you for starting this up. This website is one thing that
is needed on the web, someone with a little originality!
[…] hear it from me.” Most places won’t rent equipment to anyone that doesn’t have a northbridge commercial insurance toronto policy, so that should cut down on the attempts of bungie jumping out of bucket trucks and […]
[…] transport companies to have at least a liability and cargo coverage. What you want in this case is commercial truck insurance toronto and not a personal […]
Sólo hay algo en lo que el 76 por cien de los daneses están en contra:
el sexo de humanos con animales.
What’s up friends, how is everything, and what you wish for to
say on the topic of this piece of writing, in my view its truly awesome in support of me.
You can actually trade as little as $1 for every single
deal. Higher risks are unacceptable and should be avoided.
With this system, which is best for limiting venture, the strategist splits his stake between put and call selections.
In the trading market, binary stands for the up or the down movements of the currency,
index or stocks. You will have underlying asset options and the current price of your asset will be listed on the screen. One distinct type of options trading is the pairing method.
Teгrible,perturbé chocolat sauvеr renforcer au lieu ɗe r
Now there are some free radicals that should not only be locked up, but I dare say destroyed.
If the government can’t pay its debts we might as well throw in the towel.
With an unpredictable audit future, and regulations and compliance being a primary focus of government contracting procedures, management through Sunflower Systems solutions guarantees solutions for the present and
future of the contractor’s property organization and processes.
My web blog linda kowalski ct
What’s up, after reading this remarkable post i
am too delighted to share my knowledge here with mates.
It’s actually a cool and helpful piece of information. I’m happy that you simply shared this useful info with us.
Please stay us up to date like this. Thank you for sharing.
Some tips i have constantly told persons is that while searching for a good internet electronics retail store, there are a few factors that you have to consider. First and foremost, you want to make sure to locate a reputable and in addition, reliable store that has gotten great testimonials and classification from other individuals and market sector people. This will make sure that you are dealing with a well-known store that delivers good services and aid to their patrons. Many thanks sharing your opinions on this weblog.
There are several other marine electronics that are also required
for better communication and entertainment needs while you are on the sea.
cw Hood 32 – Want something with cleaner more traditional lines.
1) Engines: All fresh water passages should be drained
completely so no water is left in the tank.
DSL and Cable is also very dependent on distance in an effort to
deliver quick entry.
There is definately a lot to know about this issue.
I love all of the points you have made.
Hello there! I could have sworn I’ve been to this
blog before but after going through a few of the articles I realized
it’s new to me. Nonetheless, I’m certainly pleased I came across it and
I’ll be book-marking it and checking back often!
There’s definately a lot to find out about this subject.
I like all of the points you made.
Awesome blog! Do you have any recommendations
for aspiring writers? I’m hoping to start my own website soon but I’m a little
lost on everything. Would you propose starting with a free platform like WordPress or go for a paid option? There are so many
options out there that I’m completely confused ..
Any ideas? Thanks!
wonderful points altogether, you simply gained a new reader.
What might you recommend about your put up that you simply made
some days ago? Any certain?
After I initially commented I seem to have clicked the -Notify me when new comments are added- checkbox and from
now on each time a comment is added I receive four emails with the same comment.
Is there a way you can remove me from that service? Thanks!
Useful information. Lucky me I found your site accidentally, and I am surprised why
this accident did not happened in advance! I bookmarked it.
Thanks for any other informative blog. Where else could I
am getting that kind of info written in such an ideal means?
I’ve a undertaking that I am simply now operating on, and I’ve been at the look out for such information.
Thank you for the good writeup. It in reality was actually a amusement account it.
Look advanced to more added agreeable from you!
Furthermore, how could we communicate?
I have read so many content concerning the blogger lovers except this paragraph is truly
a good article, keep it up.
Hello mates, its impressive piece of writing about educationand fully
defined, keep it up all the time.
After looking into a handful of the blog posts on your web page, I honestly like your way of blogging.
I book marked it to my bookmark site list and
will be checking back in the near future. Take a look at my website too and tell me your opinion.
We absolutely love your blog and find the majority of your post’s to be just
what I’m looking for. Do you offer guest writers to write
content available for you? I wouldn’t mind creating a post or elaborating
on a lot of the subjects you write related to here.
Again, awesome website!
I was curious if you ever considered changing the structure
of your blog? Its very well written; I love what youve
got to say. But maybe you could a little more in the way of content so people could connect with it better.
Youve got an awful lot of text for only having 1 or 2 pictures.
Maybe you could space it out better?
Hello! I’m at work surfing around your blog from my new
apple iphone! Just wanted to say I love reading through your blog and
look forward to all your posts! Keep up the superb work!
Wow, marvelous weblog format! How lengthy have
you been running a blog for? you make running a blog
glance easy. The total look of your website is
great, as smartly as the content!
This post is on 12 spot in google’s search results, if you
want more visitors, you should build more backlinks to your content, there is one trick to get free, hidden backlinks from authority
forums, search on youtube; how to get hidden backlinks from forums
I don’t even know how I stopped up here, but I assumed this post was once great.
I don’t recognize who you’re however definitely you’re going to a well-known blogger
when you are not already. Cheers!
Hiya very nice site!! Guy .. Excellent ..
Superb .. I’ll bookmark your site and take the feeds additionally?
I’m happy to seek out numerous useful information here in the publish, we’d like work out
extra strategies on this regard, thanks for sharing. . . .
. .
I pay a quick visit daily a few blogs and blogs to read content, except this website presents quality based articles.