Three methods of preventing hydrate formation in pipelines and processing facilities are commonly used in our industry. These are:
1) Maintain the T & P of the system outside of the hydrate formation region.
2) Dehydrate the gas to remove the water.
3) Inhibit hydrate formation with chemical inhibitors.
Option 3 is commonly used when it is impractical or uneconomic to install dehydration facilities, typically glycol dehydration.
There are a number of chemicals used to inhibit hydrate formation, but generally fall into one of two types: equilibrium (sometimes called thermodynamic) inhibitors or kinetic inhibitors. Equilibrium inhibitors lower the equilibrium hydrate formation point and include polar chemicals such as alcohols, glycols and salts. Kinetic inhibitors (often referred to as Low Dosage Hydrate Inhibitors, LDHIs) do not change the equilibrium hydrate formation condition but instead modify the rate at which hydrates form or the ability of hydrate crystals to agglomerate into a plug that could block flow. These will not be discussed in this article and more detail can be found in June and July 2010 tip of the month.
The most commonly used equilibrium inhibitors used in the upstream and midstream sectors of the oil and gas business are: monoethylene or diethylene glycol (MEG or DEG) and methanol. In general, glycols are more commonly used in systems requiring continuous inhibition. The glycol is typically recovered, regenerated and recirculated. Methanol is more commonly used in systems that do not require continuous inhibition, i.e. systems only requiring inhibition during cold weather or upset conditions. In addition, methanol is not commonly recovered and reused. This is due to the difficulty of separation of the methanol from water. There are obviously exceptions to this, methanol is used as a continuous inhibitor in a few offshore installations and in a handful of gas processing facilities. Another significant disadvantage of methanol relative to glycol is the high methanol losses to both the liquid hydrocarbon and vapor phase.
The purpose of this article is to review experimental VLE data for methanol-hydrocarbon systems and to show correlations that may be used to estimate methanol losses to the vapor phase.
The total injection rate required to inhibit hydrate formation is the sum of the inhibitor in the
liquid water (aqueous phase) plus the inhibitor in the vapor phase plus the inhibitor in the liquid hydrocarbon phase, if any.
As described in Chapter 6, Volume of 1 of Reference [1], the Hammerschmidt [2], Nielsen and Bucklin [3], or Maddox et al. [4] equations can be used to estimate weight percent of methanol or glycol in the rich solution (aqueous phase) required to inhibit hydrate formation. The actual inhibitor injection rate to satisfy the aqueous phase inhibitor concentration needed is found by material balance and is a function of the amount of water to be inhibited as well as the lean inhibitor concentration.
Figure 6.20 on page 191 of reference [1] provides reliable estimates of vaporization loss for pressures less than about 4830 kPa (700 psia) and water phase methanol concentrations less than about 40 weight %. At higher pressures methanol losses to the vapor phase may be significantly higher than indicated in Figure 6.20, particularly at high methanol concentrations.
In this Tip Of The Month (TOTM), we will revisit Figure 6.20 of reference [1] for methanol loss to the vapor phase using the experimental vapor liquid equilibrium data reported in the Gas Processors Association Research Report 117 (GPA RR 117) [5]. The objective of this TOTM is to reproduce the same diagram covering wider ranges of pressure, temperature and weight percent of methanol in the aqueous phase. First we demonstrate the accuracy of ProMax [6] and then the polar version of Peng-Robinson [7] equation of state (PR EOS) of the same software to generate the required data. Finally, for ease of use the generated results are presented graphically.
Results and Discussion:
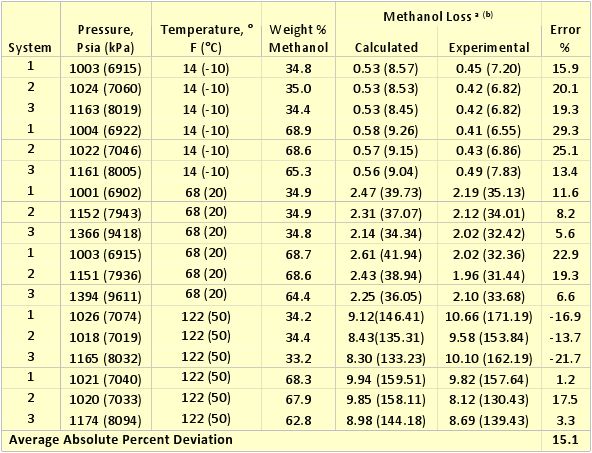
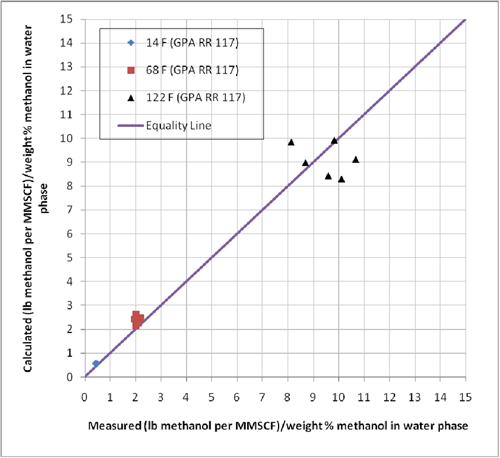
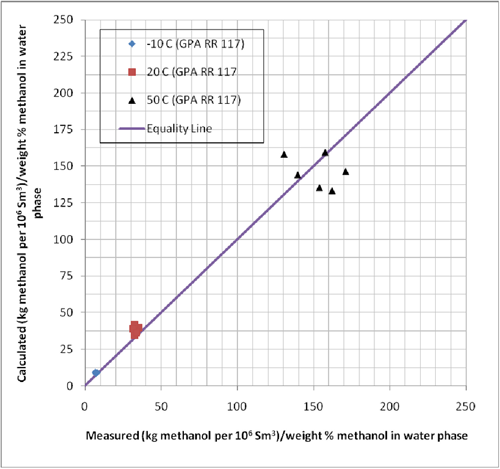
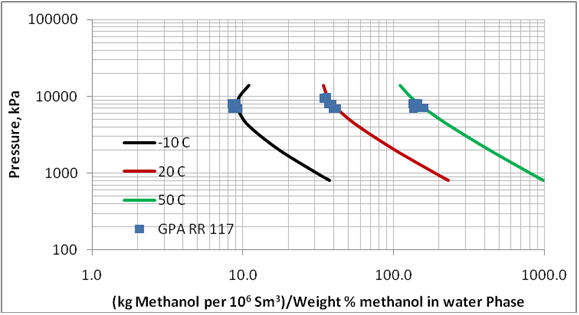
GPA RR 117 presents experimental equilibrium phase compositions for systems containing methane and n-heptane, methane and methylcyclohexane (MCH), and methane-toluene in the presence of 35 and 70 weight % methanol solutions. The experimental conditions for these data are shown in Table 1. In order to evaluate the accuracy of the ProMax software for these systems, we predicted the ratio of vapor to liquid composition in terms lbm of methanol per MMSCF/(weight % methanol in aqueous phase) or kg of methanol per 106 Sm3/(weight % methanol in aqueous phase). The results of this comparison are also shown in Table 1. The same comparison results are also shown graphically in Figures 1 and 2.
Table 1 indicates that the average absolute percent deviation for the 18 cases tested is about 15% with a maximum deviation of 23%. Considering the fact that the experimental data has some inherent error and some scatter in the data, the accuracy of ProMax is reasonably good for determination of methanol loss to the vapor phase.
Table 1. Accuracy of ProMax for calculating methanol loss to vapor phase
- Methane-n-Heptane-Methanol-Water
- Methane-Methylcyclohexane-Methanol-Water
- Methane-Toluene-Methanol-Water
- (lbm of methanol per MMSCF)/(Weight % methanol in aqueous phase)
- (kg of methanol per 106 Sm3)/(Weight % methanol in aqueous phase)
Figure 1. Comparison of predicted methanol loss to the vapor phase by ProMax with the experimental values reported in GPA RR 117.
Figure 1 (SI). Comparison of predicted methanol loss to the vapor phase by ProMax with the experimental values reported in GPA RR 117.
Figure 2. Comparison of predicted methanol loss to vapor phase by ProMax with the experimental values reported in GPA RR 117.
Figure 2 (SI). Comparison of predicted methanol loss to vapor phase by ProMax with the experimental values reported in GPA RR 117.
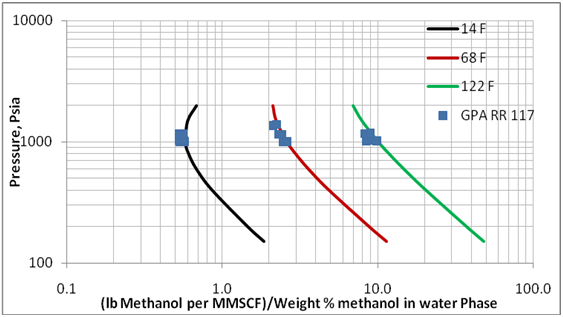
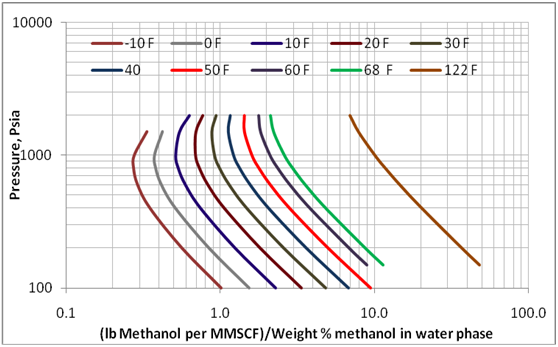
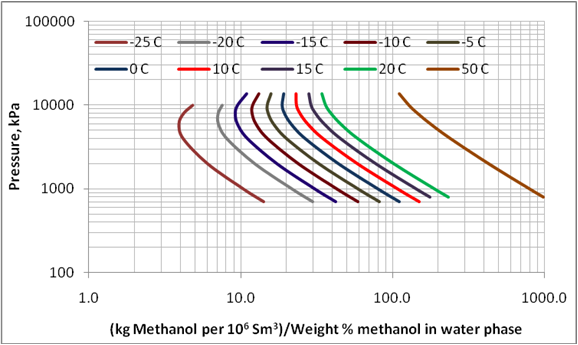
Figure 3 presents the effect of pressure and temperature on the methanol loss to the vapor phase. This diagram is generated for a mixture with total composition of 33.63 mol% methane, 22.42 mol% n-heptane, 24.95 Mol% methanol, and 19 mol % water. A three phase calculation on this mixture produces, at various pressure and temperature, vapor phases containing about 98 mol% methane and aqueous phases containing about 70 weight percent methanol. A similar diagram was generated for another mixture with composition of 29.43 mol% methane, 19.62 mol%, n-heptane, 11.86 Mol% methanol, and 39.09 mol % water. The later mixture produces an aqueous phase containing about 35 weight percent methanol. The calculated corresponding methanol losses were close for the two mixtures.
Figure 3. Variation of methanol loss to vapor phase with pressure and temperature over methanol concentration of up to 70 weight % methanol in the aqueous phase
Figure 3 (SI). Variation of methanol loss to vapor phase with pressure and temperature over methanol concentration of up to 70 weight % methanol in the aqueous phase
Conclusion:
As shown in Table 1 and Figures 1 and 2, the limited experimental data of GPA RR 117 indicate that ProMax can be used to estimate the methanol loss to the vapor phase. Therefore, this software was used to reproduce Figure 6.20 in reference 1 and presented here in this work as Figure 3. This figure covers wider ranges of pressure, temperature, and methanol weight percent (up to 70 weight %). Even though the methanol loss to the vapor phase shown on the x-axis of Figure 3 depends on the gas composition, the effect of composition is small and can be negligible for the planning purposes and facilities calculations.
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses; G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing-Special).
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By Dr. Mahmood Moshfeghian
Reference:
- Campbell, J. M. “Gas conditioning and processing, Volume 1: Fundamentals,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.
- Hammerschmidt, E. G., Ind. Engr. Chem., Vol. 26 (1934), p. 851.
- Nielsen, R. B. and R. W. Bucklin, “Why not use methanol for hydrate control?,” Hyd. Proc., Vol. 62, No. 4 (Apr. 1983), p. 71
- Maddox, R.N., M. Moshfeghian, C. H. Tu, A. Shariat, and A. J. Flying “Predicting Hydrate Temperature at High Inhibitor Concentration,” Proceeding of Laurence Reid Gas Conditioning Conference, March 4 – 6, 1991.
- Ng, H. J., Chen, C. J., and D. B. Robinson, D.B.; RR-117, “The Solubility of Methanol or Glycol in Water – Hydrocarbon Systems,” Gas Processors Association (Mar. 1988).
- ProMax 3.1, Bryan Research and Engineering, Inc, Bryan, Texas, 2010.
- Peng, D. Y., and Robinson, D. B., Ind. Eng. Chem. Fundam., Vol. 15, p. 59, 1976.