Benzene, toluene, ethylbenzene, and xylene are members of the aromatics hydrocarbon family group, often referred to as BTEX. These aromatic compounds are also belonged to the broader category of Hazardous Air Pollutants (HAPs). Benzene is a known carcinogen and has also been shown to cause blood disorders and to impact the central nervous system and the reproductive system. Toluene may affect the reproductive and central nervous systems. Ethylbenzene may have respiratory and neurological effects [1]. BTEX can be present in many natural gas streams and are partially absorbed by the solvent in glycol dehydration and amine sweetening units.
In gas treating service, methyl diethanolamine (MDEA) will absorb limited quantities of BTEX from the gas. Based on literature data, predicted absorption levels for BTEX components vary from 5 to 30% [2]. Absorption is favored at lower temperatures, higher MDEA concentrations and circulation rates. The bulk of absorbed BTEX is separated from the MDEA in the regeneration unit and leaves the system in the regenerator overhead stream which requires further treatment before being vented to atmosphere. The amount of adsorption of the BTEX is required to be known to determine the proper treating method for the overhead acid gases leaving the regenerator.
The emission of BTEX components from glycol dehydration is also regulated in most countries. In the U.S., benzene emissions are limited to 1 ton/year (900 kg/year). Mitigation of BTEX emissions is an important component in the design of a dehydration systems. Correctly estimating the quantity of absorbed BTEX and understanding the factors that affect absorption levels is critical to ensure the proper mitigation methods are provided to meet the required emission limits.
The GPA Midstream research report RR-242 [3], is an extension of several previous GPA Midstream research projects looking at the solubility of hydrocarbons in loaded and unloaded amine solutions. The previous research included research reports RR-180, 185, 195, and RR-220.
The previous projects have concentrated only on two amine systems, MDEA and DGA, both loaded and unloaded with several model hydrocarbons. RR-242 expands the current base of research data to other amines (including DEA, MEA, and a MDEA/piperazine blend), as well as measures the influence of CO2 and H2S (so called loaded amines).
Accurate hydrocarbon solubility data of RR-242 enables the development of new equation of state correlations that can be applied to the simulation of amine units (Bullin and Brown [4]). The data can be used to optimize the design and operation of amine units in which these hydrocarbons are present in the feed gas. The data will provide a basis for accurately predicting the distribution of the heavier hydrocarbons between the treated gas, the amine flash gas, and the acid gas streams. The data will provide a basis for accurately predicting emissions of these hydrocarbons from the amine unit to aid in the design and operation of these units (Moshfeghian and Hubbard [5]).
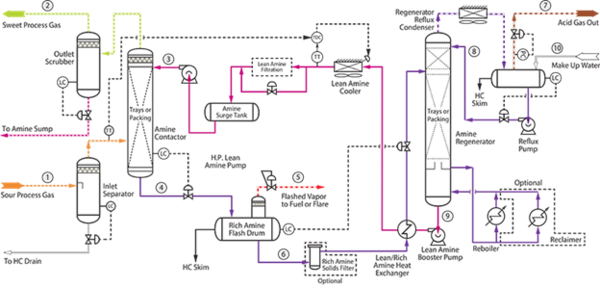
Figure 1- Process flow diagram of MDEA acid gas recovery unit [2]
This tip will focus on the following design variables:
– Solubility of selected hydrocarbons in pure water, Equation 1, and Figure 2.
– GPA Midstream RR-242 Proposed Model
– Relative solubility of benzene, toluene, and ethyl benzene (EBenzene), Figures 3, 4, and 5.
– Relative solubility of benzene, toluene, and ethyl benzene (EBenzene), Figure 6.
– Solubility of benzene, toluene, and Ebenzene in 50 wt % MDEA solution, Figure 7, and Table 1.
Solubility of selected hydrocarbons in pure water:
Solubility of selected hydrocarbons in pure water can be estimated using Equation 1 [3] or Figure 2.
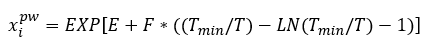 (1)
(1)
Where T is the temperature in K and the correlation parameters, E, F and Tmin, are shown in Table 1.
Table 1- Equation 1 parameters, E, F and Tmin [3].
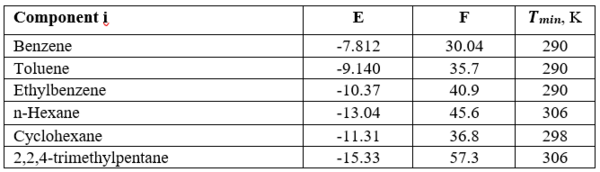
Figure 2 was generated using Equation 1 and its parameters in Table 1. The solubility of these selected hydrocarbons in pure water will be used to estimate their solubilities in amine solution in the proceeding sections.
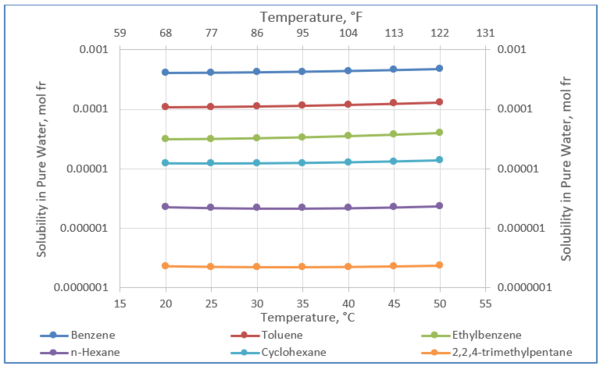
Figure 2. Solubility of selected hydrocarbons in pure water [3].
Estimation of solubility of hydrocarbons in amine solution by GPA Midstream RR-242 Model [3]
Equation 2 with its corresponding parameters will be used to estimate the solubility of selected hydrocarbons in unloaded and loaded amine solutions.
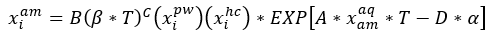 (2)
(2)
Where:
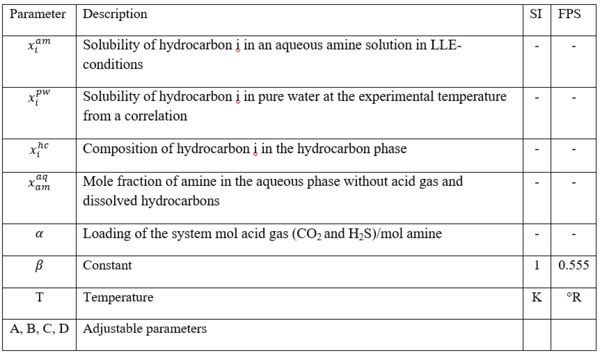
The experimental measurement data of relative solubility of benzene, toluene, and ethyl benzene (EBenzene) in 50 wt % MDEA solutions are shown in Figures 3, 4, and 5. In addition the estimated solubility data by Equation 2 is superimposed on these three figures.
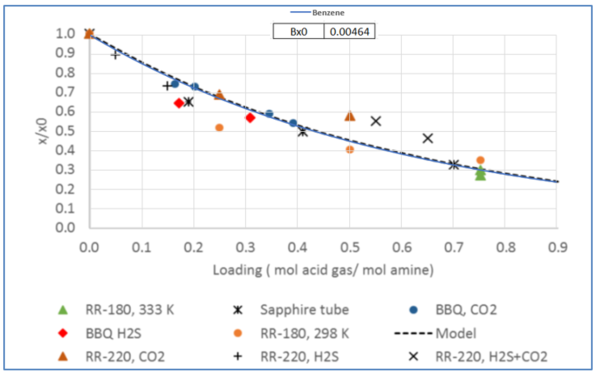
Figure 3 – Relative solubility of Benzene in 50 wt % (13.1 mol-%) MDEA-Water [Fig 16 of GPA RR 242].
Figure 4 – Relative solubility of Toluene in H2S or CO2 loaded 50 wt % (13.1 mol-%) MDEA-Water [Fig 18 -GPA RR 242].
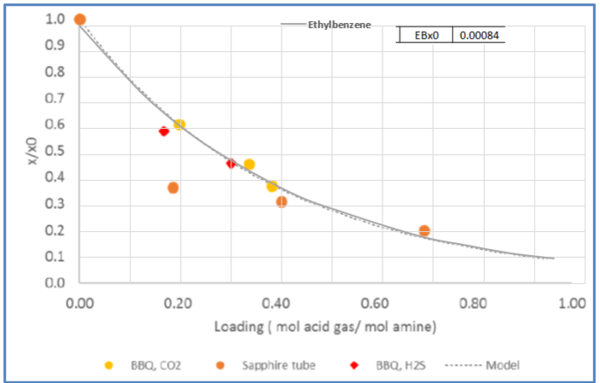
Figure 5 – Relative solubility of Ethylbenzene in CO2 loaded 50 wt % (13.1 mol-%) MDEA-Water [Fig 20-GPA RR 242].
Tables 2 and 3 indicate that estimated solubility by the model agree well with the experimental measurements.
Table 2 – Solubility of Toluene in CO2-loaded aqueous MDEA (13.13 mol-%)/water [3].
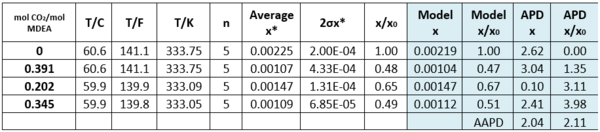
n – number of analyzed samples, x*–average, σ – standard deviation, x/x0 – soluble loaded/soluble. non-loaded
Table 3 – Solubility of Toluene in H2S-loaded aqueous MDEA (13.13 mol-%)/water [3].
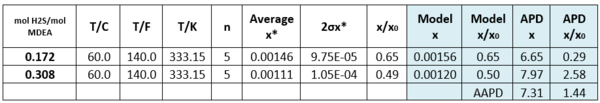
APD = Absolute percent deviation, AAPD = Average Absolute percent deviation,
Example 1
Determine the solubility of benzene in loaded 13.1 mol% (50 wt%) MDEA in water solution in terms of scf of gas/gal of solvent (std m3 of gas/m3 of solvent) at 60 °C (140 °F), 333 K (600 °R).
Rich amine solution acid gas loading, = 0.4 mol acid gases/ mole MDEA, MDEA MW =119.17
50 wt % MDEA solution density =1017.3 kg/m3 (8.49 lbm/gal)
Solution
From Fig3, for = 0.4 mol acid gases/ mole MDEA, relative solubility, x/x0= 0.53
Benzene solubility in un-loaded MDEA, x0 = 0.00464
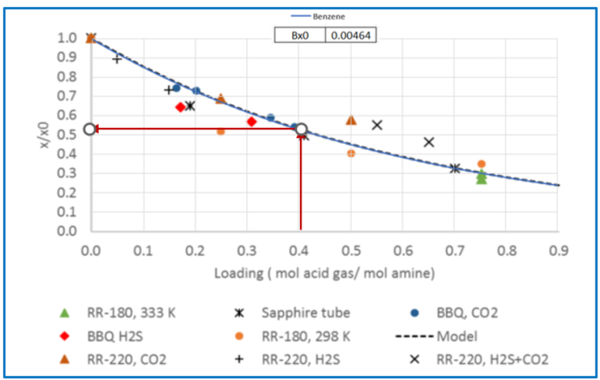
Figure 3 – Relative solubility of Benzene in 50 wt % (13.1 mol-%) MDEA-Water [Fig 16 of GPA RR 242].
Solution – SI
X = x0(x/x0) = (0.00464) (0.53kmol Ben/kmol MDEA Sol) (kmol MDEA Sol/0.131kmol MDEA)
= (0.0187 kmol Ben/kmol MDEA) (23.64 std m3/kmol Ben) (kmol MDEA/119.21 kg)
= (0.0037 std m3 Ben/kg of MDEA) (kg MDEA/2 kg MDEA + Water Sol) (1017.3 kg/m3 MDEA Sol)
= 1.88 std m3 of Ben/m3 of MDEA + water solution
Or X = (770) (x0) (x/x0) std m3 of Ben/m3 of MDEA + water solution
Solution – FPS
X = x0(x/x0) = (0.00464) (0.53 lbmol Ben/lbmol MDEA Sol) (lbmol MDEA Sol/0.131lbmol MDEA)
= (0.0187 lbmol Ben/lbmol MDEA) (379.5 scf/lbmol Ben) (lbmol MDEA/119.21 lbm)
= (0.0598 scf Ben/lbm of MDEA) (lbm MDEA/2 lbm MDEA + Water Sol) (8.49 lbm/gallon MDEA Sol)
= 0.25 scf of Ben/gallon of MDEA + water solution
Or X = (103) (x0) (x/x0) scf of Ben/gallon of MDEA + water solution
For simplicity and easiness of reading the figures, using the model (Equation 2) similar charts like Figures 6 and 7 or Table 4 can be generated.
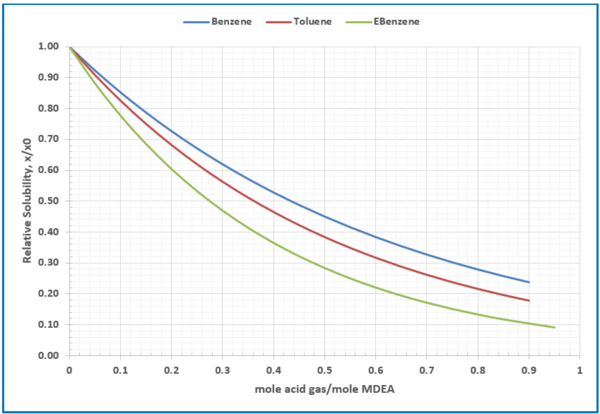
Figure 6 – Relative solubility of benzene (Fig 3), toluene (Fig 4), and Ethylbenzene (Fig 5) in loaded 50 wt % (13.1 mol-%) MDEA-Water
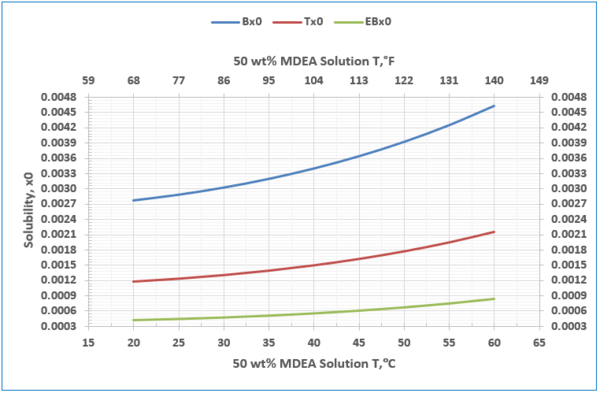
Figure 7- Solubility of benzene, toluene, and ethyl benzene in unloaded 50 wt % MDEA solution
Table 4- Solubility of benzene, toluene, and ethyl benzene in unloaded 50 wt % MDEA solution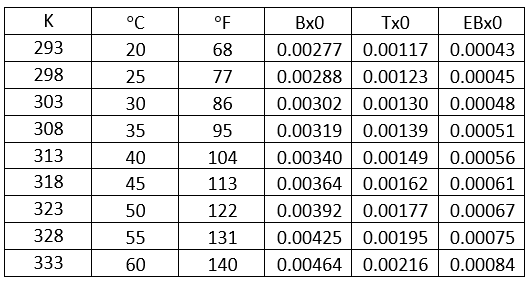
Figure 8 shows the influence of amine solution concentration and acid gas loading on solubility of toluene in 50 wt% and 25 wt% MDEA solution at 100 °F (38 °C)
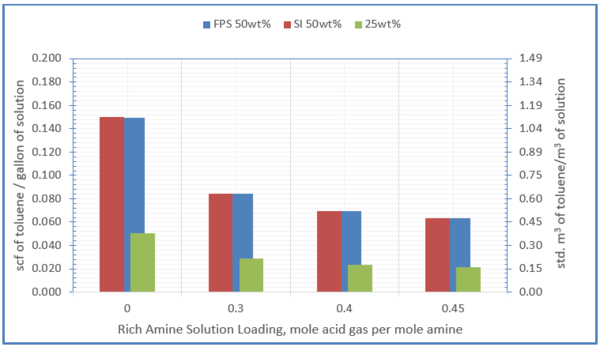
Figure 8- solubility of toluene in 50 wt% and 25 wt% MDEA solution at 100 °F (38 °C)
From the RR-242 developed model based on the experimental results; several conclusions can be drawn.
1. The solubility of the hydrocarbons depends strongly on the amine concentration in the solvent, with the solubility increasing exponentially with increasing molar concentration of the amine in the aqueous solution (Figure 8).
2. The solubility of the hydrocarbons increases with temperature (Figure 7 and Table 4).
3. The solubility of the hydrocarbons depends strongly on the acid gas loading (decreased solubility at higher loading, Figure 8.)
4. The solubility for CO2 loaded amines are found to be similar to the solubility for H2S loaded amines.
5. Finally, the ratio of hydrocarbon solubility of the loaded solvent to the unloaded solvent is found to be similar at a given acid gas loading.
Summary
Figures 6 and 7 present a simple tool for quick estimation of solubility of BETX compounds in MDEA gas treating process. For the example considered in this tip, the estimated solubility for each of BETX compounds matched well with the experimental results.
To learn more about similar cases and how to minimize operational problems, we suggest attending ourG4 (Gas Conditioning and Processing),G5 (Advanced Applications inGas Processing), http://www.jmcampbell.com/co2-surface-facilities-pf81.php andPF49 (Troubleshooting Oil & Gas Processing Facilities),courses.
By: Mahmood Moshfeghian, Ph.D.
and Kindra Snow-McGregor, P.E.
Reference:
1. http://www.earthworksaction.org/BTEX.cfm, 2011.
2. Campbell, J. M. “Gas conditioning and processing, Volume 2: The Equipment Modules,” 9th Edition, 2nd Printing, Editors Hubbard, R. and Snow–McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, 2014.
3. Uusi-Kyyny, P., Pakkanen, M., Richon, D., Ionita, S., Ogunrobo, E., Alopaeus, V., RR-242, “Solubility of Hydrocarbons in Amine Treating Solutions”, GPA Midstream Association, Tulsa, OK, 2019.
4. Bullin, J. A., Brown, W.G., Hydrocarbons and BTEX Pickup and Control from Amine Systems”, 83rd Gas Processors Association Annual Convention, Mar. 2004.
5. Moshfeghian, M. and R.A. Hubbard, “Quick Estimation of Absorption of Aromatics Compounds (BTEX) in TEG Dehydration Process”, 3rd International Gas Processing Symposium, March 5-7, Doha, Qatar, 2012.
