Equations of state (EoS) are used in commercial simulation software for predicting phase behavior and thermodynamic properties. The cubic equations of state (EoS) give relatively accurate results for predicting vapor-liquid equilibria, especially for non-polar or slightly polar systems. Furthermore, these equations can be used to accurately predict vapor densities, enthalpy and entropy. These advantages encourage the researchers to augment EoS ability more than before, especially liquid density, although their accuracy for liquid density prediction is generally poor. The popular EoSs such as Soave-Redlich-Kwong (SRK) [1] and Peng-Robinson (PR) [2] predict liquid density with an average absolute error of about 8%, much higher than several good density correlations. This large magnitude of error is not acceptable by industry; therefore, they are not used for this purpose. In order to overcome this deficiency, volume translated methods have been developed.
In the March 2011 tip of the month (TOTM) we studied a constant volume translation of liquid density Method presented by Peneloux et al. [3] and demonstrated its application for hydrocarbons such as pure methane, n-pentane, decane, pentadecane and carbon dioxide. Considerable improvements, specifically for the low temperature range (T r < 0.8), of saturated specific volume (or liquid density) predicted by PR and SRK were obtained. On the other hand, the constant volume shift fails near the critical temperature, because the slope of volume with respect to temperature greatly increases in this region.
Since Peneloux et al. presented their constant volume translation (shift) method in 1982, several temperature dependent volume correction methods [4-11] have been proposed. In this TOTM we will demonstrate application and accuracy of some of these methods to predict liquid density of common hydrocarbons and non-hydrocarbons in gas processing. We will compare their accuracy against both experimental data and a few correlations.
- CorrelationsThe following correlations were used in this study.
- COSTALD, 1979: The COSTALD correlation by Hankinson and Thomson [12] requires two parameters:
 SRK, the optimized value of the acentric factor based on the SRK equation of state (EoS) and; V*, the pure component characteristic volume.
SRK, the optimized value of the acentric factor based on the SRK equation of state (EoS) and; V*, the pure component characteristic volume. - RSD, 1972: Spencer and Danner [13] improved the liquid density correlation of Rackett [14]. The improved correlation for saturated liquid density calculation requires only ZRA, the improved compressibility factor.
- NM, 1998: Nasrifar and Moshfeghian [15, 16] presented an equation and a set of mixing rules for predicting the liquid density of pure refrigerants and liquefied natural gas.
- COSTALD, 1979: The COSTALD correlation by Hankinson and Thomson [12] requires two parameters:
- Volume Translated EoS Methods The following volume translated (shift) methods were used in this study.
- PRF, 1982: Peneloux et al. [3] proposed the following constant volume shift correction.
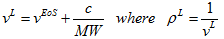 (1)In the above equation, VL is the corrected liquid specific volume, VEoS is the liquid specific volume calculated by the EoS, MW is the molecular weight, pL is the liquid density, and the correction term or volume shift factor “c” is determined from experimentally measured liquid density. The volume shift factor is normally regressed against several data points. In the absence of experimentally regressed value, it can be estimated as follows:
(1)In the above equation, VL is the corrected liquid specific volume, VEoS is the liquid specific volume calculated by the EoS, MW is the molecular weight, pL is the liquid density, and the correction term or volume shift factor “c” is determined from experimentally measured liquid density. The volume shift factor is normally regressed against several data points. In the absence of experimentally regressed value, it can be estimated as follows: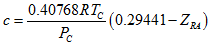 (2)where ZRA, is the Rackett [15] parameter, R is the gas constant, and TC and PC are the critical temperature and pressure, respectively.We determined the optimum value of “c” for each compound by the procedure described in
(2)where ZRA, is the Rackett [15] parameter, R is the gas constant, and TC and PC are the critical temperature and pressure, respectively.We determined the optimum value of “c” for each compound by the procedure described in
the March 2011TOTM. - MT, 1990: Magoulas and Tassios [4] temperature dependent correction factor is calculated as follows:
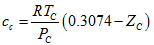 (3)
(3)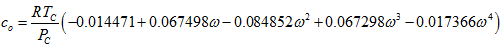 (4)
(4)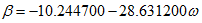 (5)
(5)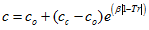 (6)We will refer to this method as MT-VTPR.
(6)We will refer to this method as MT-VTPR. - TC, 1998: Tsai and Chen [5] temperature dependent correction factor is calculated as follows:
 (7)
(7)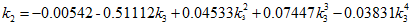 (8)
(8)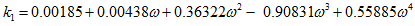 (9)
(9)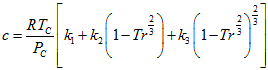 (10)
(10) - AG, 2001: Ahlers and Gmehling [6] temperature dependent correction factor, c, is calculated as follows:
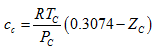 (12)
(12)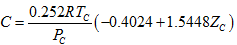 (13)
(13)
- LD, 2005: Lin and Duan [7] presented a temperature dependent factor, c, as follows:
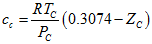 (18)
(18)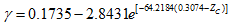 (19)
(19)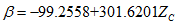 (20)
(20)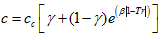 (21)
(21)
- PRF, 1982: Peneloux et al. [3] proposed the following constant volume shift correction.
In the above equations, Tr is the reduced temperature, ω is acentric factor, TC, PC and ZC are critical temperature, pressure and compressibility factor, respectively. The correction term, c, from the above methods is substituted in equation 1 to calculate the corrected density.
Results and Discussion:
A simple MathCad program was written to perform all of the calculations based on the above methods. We applied the preceding methods to several pure compounds shown in Table 1. The reduced temperature (Tr) and number of points (N) for each compound are also shown in Table 1. This Table presents the summary of the error analysis for different methods for the pure compounds. As can be seen in Table 1, these generalized temperature dependent volume shift methods improve the accuracy but yet not as good as the generalized correlation methods shown in the last three column of Table 1.
Table 1. Summary of error analysis for different methods studied
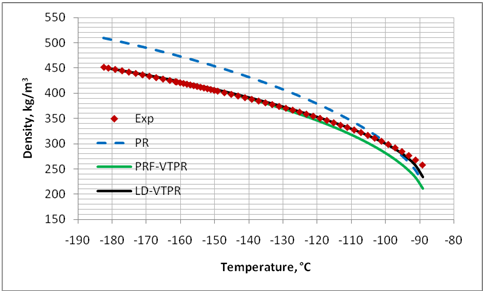
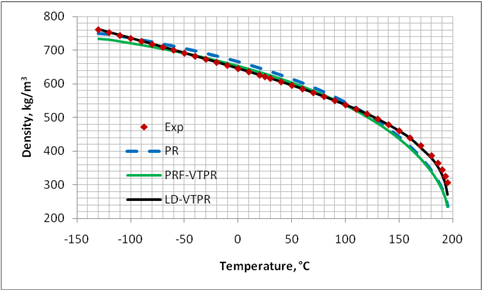
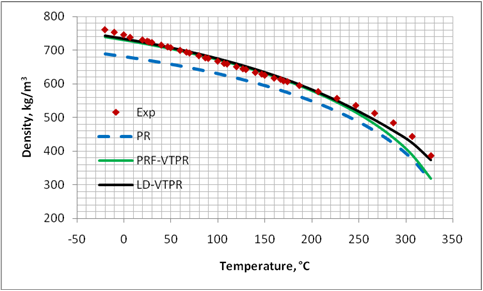
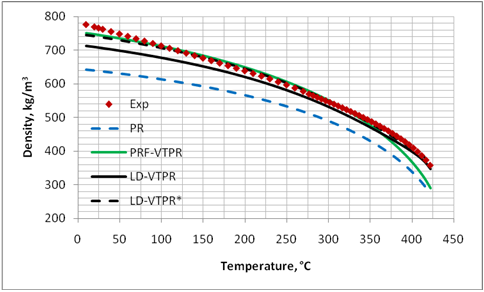
Figures 1 through 4 present graphical comparisons between the predicted and experimental [17] liquid density values of methane, n-pentane, decane and pentadecane; respectively. Similar trends were observed for the other compounds shown in Table. For clarity, only the results for PR EoS, PRF-VTPR (constant volume shift) and LD-VTPR (temperature dependent volume shift) are presented in these figures. A much better accuracy is obtained near the critical region by applying the temperature dependent volume shift.
Figure 1. Comparison of predicted liquid density of CH4 by PR EoS, volume translated PRF-VTPR and LD-VTPR against experimental data [17]
Figure 2. Comparison of predicted liquid density of C5H12 by PR EoS, volume translated PRF-VTPR and LD-VTPR against experimental data [17]
Figure 3. Comparison of predicted liquid density of C10H22 by PR EoS, volume translated PRF-VTPR and LD-VTPR against experimental data [17]
Figure 4. Comparison of predicted liquid density of C15H32 by PR EoS, volume translated PRF-VTPR and LD-VTPR against experimental data [17]
In order to show the sensitivity of the LD-VTPR method and the applicability of tuning its parameters, the ZC value for pentadecane was changed from 0.243, represented by the solid black curve in Figure 4, to 0.231 which is represented by the dashed black curve in Figure 4. The curve for ZC=0.231 is labeled as LD-VTPR*. This sensitivity is used for practical applications to tune the volume translated model parameters (e.g. ZC) to match the predicted liquid density with the experimentally measured data.
Table 1 indicates that considerable improvements are obtained by applying temperature dependent volume shift corrections to the liquid specific volume (or liquid density) near the critical point region. However, the accuracy of the COSTALD, RSD and NM correlations are still by far much better than the volume translation applied to these two EoSs. As shown in Figure 4, further improvement of volume shift methods are obtained by tuning the parameters of volume shift methods with experimental measurement.
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing-Special).
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
A free copy of the MathCad Version 14 file showing the calculations steps for methane is available upon request.
By Dr. Mahmood Moshfeghian
Reference:
- Soave, G., Chem. Eng. Sci., Vol. 27, pp. 1197-1203, 1972.
- Peng, D. Y., and Robinson, D. B., Ind. Eng. Chem. Fundam., Vol. 15, p. 59, 1976.
- Peneloux, A. E., Rauzy, E., and Freze, R., Fluid Phase Equilib., Vol. 8, pp. 7-23, 1982.
- Magoulas, K. and D. Tassios, J. of Fluid Phase Equilibria, Vol. 56, pp. 119-140-445, 1990.
- Tsai, J. and Y.P. Chen, J. of Fluid Phase Equilibria, Vol. 145, pp. 193-215, 1998.
- Ahlers, J. and J. Gmehling, J. of Fluid Phase Equilibria, Vol. 191, pp. 177-188, 2001.
- Lin, H. and Y.Y. Duan, J. of Fluid Phase Equilibria, Vol. 233, pp. 194-203, 2005.
- Ji, W.R. and D.A. Lempe, J. of Fluid Phase Equilibria, Vol. 130, pp. 49-63, 1997.
- Pfohl, O., J. of Fluid Phase Equilibria, Vol. 163, pp. 157-159, 1999.
- Frey, F., Augustine, C., Ciccolini, R.P., Paap, S., Modell, M., and J. Tester, , J. of Fluid Phase Equilibria, Vol. 260, pp. 316-325, 2007.
- Frey, F., Modell, M., and J. Tester, J. of Fluid Phase Equilibria, Vol. 279, pp. 56-63, 2009.
- Hankinson, R. W., Thomson, G. H., AIChE J., Vol. 25, no. 4, pp. 653-663, 1979.
- Spancer, C. F., and Danner, R. P., J. Chem. Eng. Data, vol. 17, no. 2, pp. 236-241, 1972.
- Rackett, H. G., J. Chem. Eng. Data, vol. 15, No. 4, pp. 514-517, 1970.
- Nasrifar, Kh. and Moshfeghian, M., Fluid Phase equilibria Vol. 153, 231-242, 1998.
- Nasrifar, Kh. and M. Moshfeghian, J. of Fluid Phase Equilibria, Vol. 158-160, pp. 437-445, 1998.
- Vargaftik, N.B., Handbook of Physical Properties of Liquids and Gases (Pure Substances and Mixtures), 2nd ed., English Translation, Hemisphere Publication, 1975.


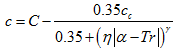
Appreciate your site. Appreciatve for the stuff on here. Anticipating for what’s new!
I love reading these articles because they’re short but infivmatore.
I just added this web site to my rss reader, great stuff. Can’t get enough!