In the past tips of the month (October, November, December 2007, January 2011, February, September 2014. And April 2015), we studied in detail the water phase behaviors of sweet and sour natural gases and acid gas systems. We evaluated the accuracy of different methods for estimating the water content of sweet and sour natural gases as well as for acid gas systems. In addition, correlations to estimate vapor water content of lean sweet and sour gases were presented.
For normal gas conditioning processes, the water vapor content of natural gases in equilibrium with water is commonly estimated from McKetta and Wehe [1] based charts like Figure 6.1 of Campbell book [2] or Figure 20.4 of Gas Processors and Suppliers Association [3]. This tip presents a chart for estimating methane gas water content at low temperatures, e.g. cryogenic processes.
Hydrate formation is a kinetic (time dependent) process. During this transient “hydrate formation period” the liquid water present is termed “metastable liquid.” Metastable water is liquid water that, at equilibrium, will exist as a hydrate. At temperatures below the hydrate temperature of the gas, the “condensed” phase will be a solid (hydrate). The water content of a gas in equilibrium with a hydrate will be lower than equilibrium with a metastable liquid. The water content of gases in the hydrate region is a strong function of composition. Page 153 of Campbell book [2] provides more detail.
Figures 1A (SI, international system of units) and 1B (FPS field system of units) present a chart for methane gas water content for temperature range of 0 °C to -80 °C (32 °F to -110 °F) and pressures of 0.345 MPa, 3.45 MPa, and 6.9 MPa (50 psia, 500 psia, and 1000 psia). The chart is based on the experimental data reported in GPA-Midstream RR 187 [4] for pressures of 3.45 and 6.9 MPa (500 and 1000 psia) and the results generated using ProMax [5] freeze model (Solid-Vapor-Equilibrium) at low pressure of 0.345 MPa (50 psia).
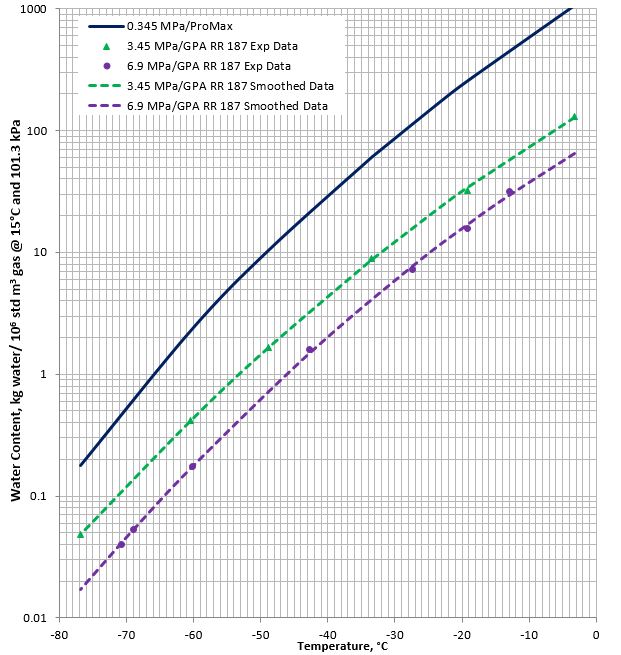
Figure 1A (SI). Low temperature methane gas water content
(Dashed lines are GPA-Midstream RR 187 data [4], solid line predicted using ProMax [5])
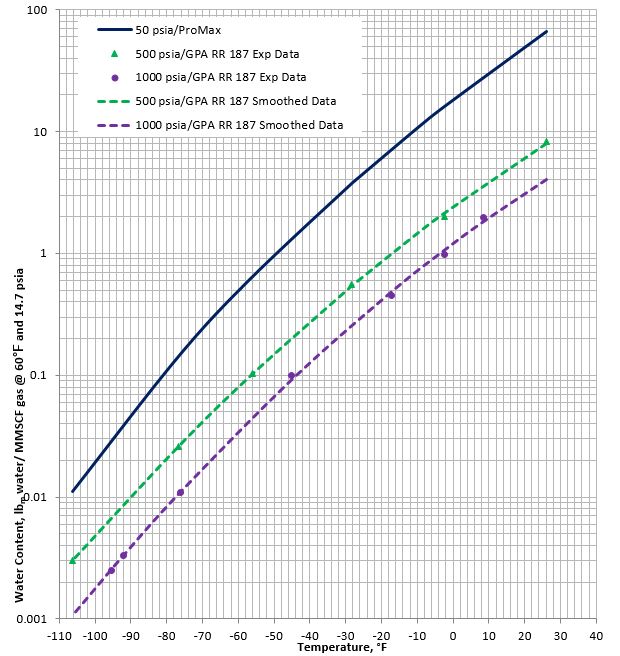
Figure 1B (FPS). Low temperature methane gas water content
(Dashed lines are GPA-Midstream RR 187 data [4], solid line predicted using ProMax [5])
Based on the methane hydrate formation diagram shown in Figure 2, the above-mentioned low temperatures are generally below the hydrate formation temperature at the corresponding pressures. Therefore, the water contents reported in Figures 1A and 1B are in equilibrium with hydrates.
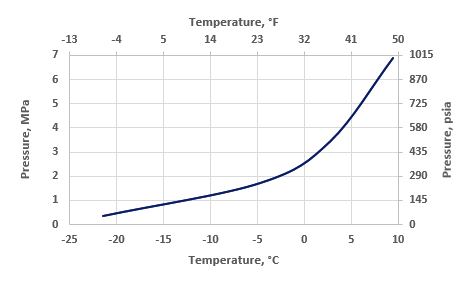
Figure 2. Methane hydrate formation temperature as a function of pressure
Summary
Figure 1 presents a simple chart to estimate methane gas water content in equilibrium with hydrates. The water content of gases in the hydrate region is a strong function of composition. Where experimental data is unavailable, utilization of an EOS-based correlation that has been tuned to empirical data can provide an estimate of water content in equilibrium with hydrates.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing), G5 (Practical Computer Simulation Applications in Gas Processing), and PF49 (Troubleshooting Oil & Gas Processing Facilities), courses.
PetroSkills offers consulting expertise on this subject and many others. For more information about these services, visit our website at petroskills.com/consulting, or email us at consulting@PetroSkills.com.
By: Dr. Mahmood Moshfeghian
Reference:
- McKetta, J. J., and Wehe, A. H., “Use This Chart for Water Content of Natural Gases,” Petroleum Refiner (Hydrocarbon Processing), Vol. 37, No. 8, p. 153, August 1958.
- Campbell, J.M., Gas Conditioning and Processing, Volume 1: The Basic Principles, 9th Edition, 2nd Printing, Editors Hubbard, R. and Snow–McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, 2014.
- GPSA Engineering Data Book, Section 20, Volume 2, 13th Edition, Gas Processors and Suppliers Association, Tulsa, Oklahoma, 2012.
- Song, K. Y., Yarrison, M., Kobayashi, R., and W. Chapman, “Low Temperature V-L-E Data for Water, CO2, and Light Hydrocarbon Systems,” Gas Processors Association Research Report RR 187 Tulsa, Oklahoma, 2005.
- ProMax 4.0, Bryan Research and Engineering, Inc., Bryan, Texas, 2017.
