In the last two “Tip of the Month” we briefly discussed the importance of liquid density for process simulation and equipment design. Three different methods were introduced to compute liquid density. The methods were (a) charts and monographs, (b) correlations and (c) EoS or volume translated EoS. We also presented a comparison between accuracy of different correlations and EoS methods and provided guidelines for using correlations.
In this “Tip of the Month”, we will present a comparison between accuracy of HYSYS [1] and ProMax [2] process simulation packages which normally use correlations for liquid density calculations. For HYSYS we used the “Smooth Liquid Density” option and in ProMax there are two options of COSTALD [3, 4] and Rackett [5] but we only used the COSTAD method for the reasons discussed in the last tip of the month. Both of these methods are discussed in Chapter 3 of JMC Volume 1 of Gas Conditioning and Processing Book [6]. The focus will be on the mixture of light hydrocarbons which have wider applications in gas industry. We will provide guidelines to use these process simulation programs effectively. In this study we have used the experimental data reported in GPA Research Report RR-147 [7].
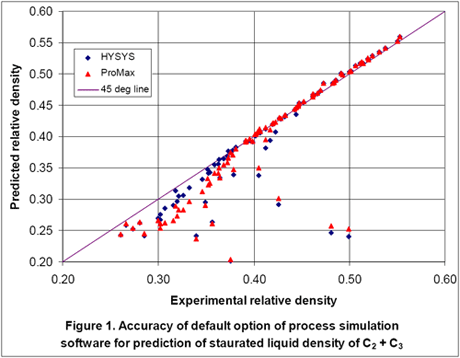
We predicted the saturated liquid density of the ethane-propane mixture for the conditions reported in the GPA research report using the default option of HYSYS and ProMax. For the default option for each set of experimental conditions, we entered temperature, pressure, composition and total number of moles (100 moles was used for all cases). In Figure 1, the predicted results for 90 points are plotted as a function of the experimental values.
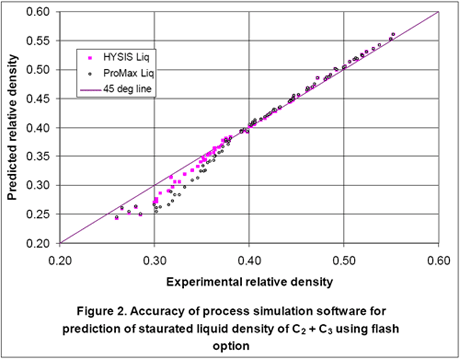
As can be seen in this figure, for several points the errors are very large. These large errors are due to the fact that for these points the process simulators predict partially vaporized systems and the reported densities are for a two phase mixture and not for the actual liquid mixture as reported experimentally. Therefore, we performed a flash calculation for each experimental point, separated the gas from the feed and predicted the density for the resulting liquid stream and re-plotted the results in Figure 2. This causes some changes in composition but Figure 2 indicates that considerable improvement in accuracy is obtained by degassing the feed stream.
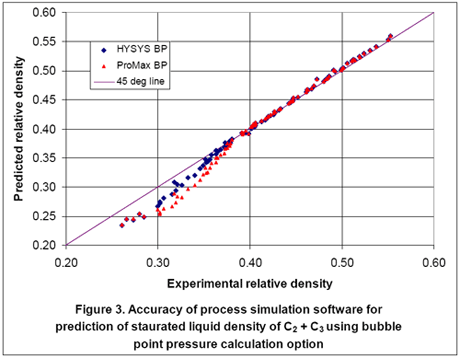
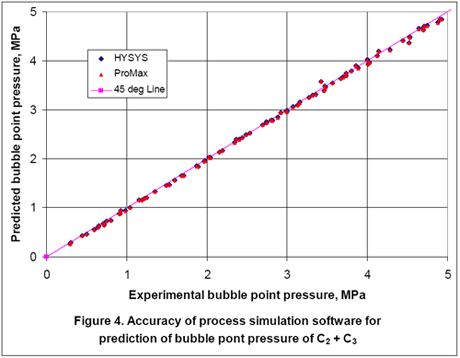
Since the reported experimental data were at saturated liquid conditions, a second option is to predict the liquid density using the bubble point option. For this option, we entered temperature, vapor fraction of zero, composition of components, and 100 moles for total feed. By performing bubble point calculation, the liquid density and bubble point pressure were calculated. Figures 3 and 4 show the accuracy of HYSYS and ProMax in predicting the liquid densities and bubble point pressures, respectively. Again, quite an improvement is obtained by performing bubble point calculation to obtain the liquid density.
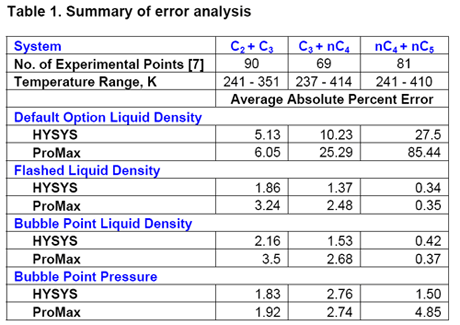
We repeated similar calculations for propane-normal butane and normal butane-normal pentane mixtures and have summarized in Table 1 the error analysis for different options using the simulation softwares.
Table 1 indicates that if the default option of HYSYS and ProMax are used, the calculated liquid density may contain a large error. On the other hand, when the mixture was flashed and the vapor was removed the calculated density was more accurate. Finally, calculating the liquid density using bubble point calculation yields more accurate density; however, the pressure may deviate slightly from the specified system pressure. The deviation of pressure does not cause a major concern because the pressure effect on liquid properties is not that much and more often it is ignored.
To learn more about liquid density and its impact on facilities calculation, design and surveillance, refer to JMC books and enroll in our G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special) courses.
By Dr. Mahmood Moshfeghian
Reference:
- HYSYS, version 2004.2, Aspen Technology Inc., Cambridge, Massachusetts, 2005.
- ProMax, version 1.2, Bryan Research & Engineering Inc, Bryan, Texas, 2005.
- Hankinson, R. W.; Thomson, G. H. A new correlation for saturated densities of liquids and their mixtures. AIChE J. 1979, 25, 653.
- Thomson, G. H.; Brobst, K. R.; Hankinson, R. W. An improved correlation for densities of compressed liquids and liquid mixtures. AIChE J., 28, 671, 1982
- Rackett, H. G. Equation of state for saturated liquids. J. Chem. Eng. Data, 15, 514, 1970
- Campbell, J. M. “Gas conditioning and processing, Volume 1: Fundamentals,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.
- Holcomb, C.D., Magee, J.W., and W.M. Haynes, “Density Measurements on Natural Gas Liquids,” Gas Processor Associations, RR-147, Tulsa, 1995.