Because phase envelope generation and its impact on design and performance of gas processing plants is so important it has been the topic of several Tips Of The Month (TOTM). As emphasized by Rusten et al. [1], there are several challenges that have to be addressed in order to succeed with the phase envelope modeling of real natural gases. The most important are:
- Sampling procedures
- Sample preparations
- Chromatographic gas analysis. A detailed composition is required for satisfactory input to thermodynamic models
- Thermodynamic models that correctly predict the phase envelope
In this TOTM we will demonstrate the impact of thermodynamic modeling for rich gases in the dense phase region. For a discussion on the dense phase, please see the January 2010 TOTM. The value of the dense phase viscosity is very similar to gas phase viscosity. The dense phase density is closer to the liquid phase density. Therefore, it has become attractive to transport rich natural gas in the dense phase region. In October 2005 we discussed several methods of C7+ (heavy ends) characterization and checked the accuracy of several methods and presented tips to improve the accuracy of each method. These methods are presented briefly below. For more detail, please refer to Gas Conditioning and Processing, Volume 3, Advanced Techniques and Applications [2].
Method A: The C7+ is treated as a single hypothetical component based on its molecular weight (MW) and specific gravity (SG). The normal boiling point is predicted; the critical temperature, critical pressure, and acentric factor are also predicted using correlations similar to the ones by Riazi and Duabert [3].
Adjusting MW (or Tc) in Method A: By adjusting the molecular weight of the C7+ fraction we can closely match the measured dew point. The critical temperature (Tc) can also be adjusted to make the phase envelope curve pass through the measured dew point. The Tc adjustment is preferred because less work is involved to match the calculated and experimental values.
Method B: The C7+ is broken into Single Carbon Numbers (SCN) ranging from SCN 7 to SCN 17+ using the exponential decay procedure presented by Katz [4] and applied by others [5-7].
Method C: The large number of SCN components of Method B may be lumped into 4 cuts. The properties of the lumped cuts are estimated from the individual SCN components.
Method D: This method is similar to Method B except that 12 normal parafins (alkanes) are used to represent the C7+instead of SCN components. The advantage of this method is that n-alkane components are readily available in many commercial software packages but the SCNs may not be.
Tuning MW in Method D: The distribution (i.e. mole %) of the alkane part of the C7+ depends on the assumed value of the C7+ MW.
Tuning the binary interaction parameters, kij, in Methods B and C: A common correlation to estimate the binary interaction parameter is:
In the above equation, νci and νcj represent the critical volumes of components i and j, respectively. The default value of exponent n is normally set to 1.2 but it can be used as a tuning parameter to match the experimentally measured dew point.
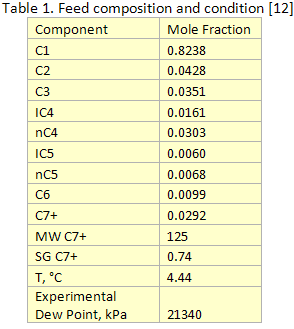
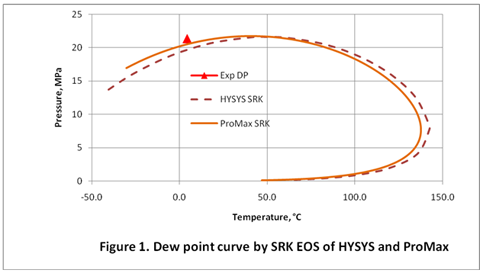
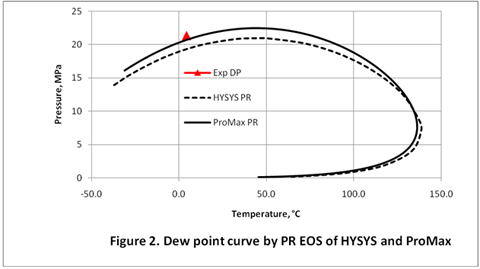
In this TOTM we will generate the dew point curve for the rich gas shown in Table 1 using the C7+ characterization methods described above. The dew point curve portion of the phase envelope for this gas was generated using both HYSYS [8] and ProMax [9] simulation software by the Soave-Redlich-Kwong (SRK) [10] (Figure 1) and Peng-Robinson (PR) [11] (Figure 2). The experimentally measured dew point pressure [12] is also show in these two figures as a red triangle.
Figures 1 and 2 were generated using a single C7+ cut with the relative density and molecular weight shown in Table 1. Other required properities were estimated using the default options of the simulation software. As can be seen in these figures using the PR Equation of State with ProMax gives the closest prediction of the experimentally measured dew point. As decribed above the MW can be adjusted to match experimantal and calculated data.
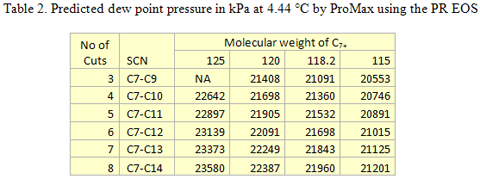
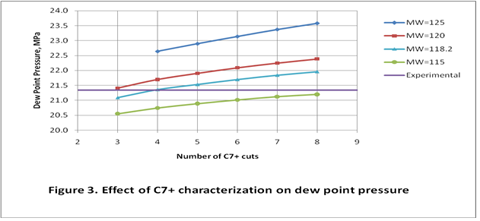
The single carbon number (SCN) analysis as described in Method B above was used for further tuning of the thermodynamic model, The predidicted dew point pressures for the different cases studied here are shown in Table 2. Figure 3 demonstrates the same information graphically.
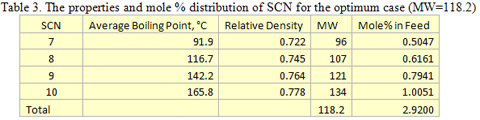
Using Method B, the experimental dew point is most closely represented using four SCNs with a combined molecular weight of 118.2. The properties and mole percent distribution of these four SCN components for the optimum case are given in Table 3.
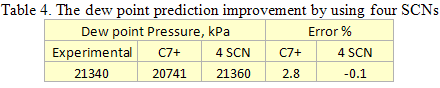
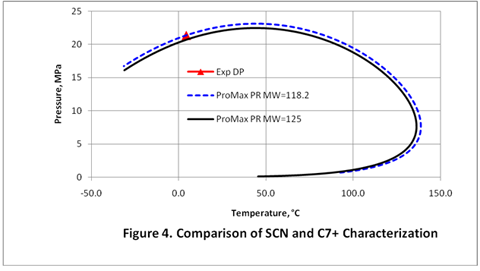
Table 4 shows the improvement made in the dew point prediction by using four SCNs with a modified molecular weight of 118.2 instead of a single C7+ cut. The ProMax PR EOS is used for both cases. The predicted dew point curves for these two cases can be seen in Figure 4.
As can be seen in Figure 4, proper characterization of the heavy components (see Tables 3 and 4) can improve the quality of the phase envelope and match the experimentally measured dew point in the dense phase region. For a detailed discussion of this topic, the readers may refer to the Rusten et al. paper [1].
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses; G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special).
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- Rusten, B.H., Gjertsen, L.H., Solbraa, E., Kirkerød, T., Haugum, T. and Puntervold, s., “Determination of the phase envelope – crucial for process design and problem solving,” presented at the 87th GPA National Convention, Grapevine, 2008
- Maddox, R. N. and L. Lilly, “Gas Conditioning and Processing, Computer Applications for Production/Processing Facilities,” John M. Campbell and Company, Norman, Oklahoma, 1995.
- Riazi, M.R. and T.E. Daubert, Hydr. Proc. P. 115, (March) 1980
- Katz, D. J. Petrol. Technol., 1205-1214, (June) 1983.
- Whitson, C. H. SPE J., 683-694, (August) 1983
- Starling, K. E. Presented at the American Gas Association Operations Conference, Orlando, FL, April 27-30, 2003
- Moshfeghian, M., Maddox, R.N., and A.H. Johannes, “Application of Exponential Decay Distribution of C6+ Cut for Lean Natural Gas Phase Envelope,” J. of Chem. Engr. Japan, Vol 39, No 4, pp.375-382, 2006
- ASPENone, Engineering Suite, HYSYS Version 7.0, Aspen Technology, Inc., Cambridge, Massachusetts U.S.A., 2009.
- ProMax®, Bryan Research & Engineering Inc, Version 3.2, Bryan, Texas, 2009
- Soave, G., Chem. Eng. Sci. 27, 1197-1203, 1972.
- Peng, D.,Y. and D. B. Robinson, Ind. Eng. Chem. Fundam. 15, 59-64, 1976.
- Sage, B.H, and R.H. Olds, AIME 170, 156–173, 1947.

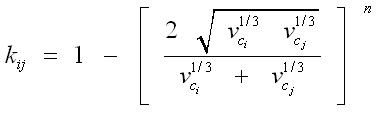
you can tune C6+ weight fractions in several ways, a limit with these methods is that a complete set of vle points is required,
for phase diagrams in addition to Promax and Hysys I would mention also Prode Properties, see http://www.prode.com for a free copy.