Liquid density is needed for process simulation and equipment design. For example, accurate predictions of liquid density are needed for calculating the pressure drop in piping/pipeline and vessel sizing. Accurate liquid density is also essential for custody transfer.
In November 2006, December 2006 and January 2007 tips of the month (TOTM), we presented an overview of different methods and tools for predicting liquid densities. The methods for liquid density prediction include but are not limited to the following.
- Generalized Charts
There are several generalized charts for predicting the liquid density of petroleum fluids and hydrocarbons [1]. The charts normally present the relative density of paraffinic hydrocarbon mixtures at their boiling point or bubble point temperature and pressure. These charts apply to mixtures as well as pure components. Alignment points for paraffinic hydrocarbon mixtures and pure components are located according to their molecular weight. The accuracy of these charts is generally within 3 % of the measured values. However, the accuracy is somewhat less for mixtures with molecular weights less than 30 where temperature is low, and where the methane content is high or pseudo-reduced temperatures are above 0.9 [2].
- Correlations
In order to calculate liquid density reliably, several correlations such as: COrresponding STAte Liquid Density (COSTALD), modified Rackett equation by Spencer and Danner (RSD), and Nasrifar-Moshfeghian (NM) have been developed.
COSTALD: The COSTALD correlation by Hankinson and Thomson [3] requires two parameters: wSRK, the optimized value of the acentric factor based on the SRK equation of state (EoS) and; V*, the pure component characteristic volume.
RSD: Spencer and Danner [4] improved the liquid density correlation of Rackett [5]. The improved correlation for saturated liquid density calculation requires only ZRA, the improved compressibility factor.
NM: Nasrifar and Moshfeghian [6] presented an equation and a set of mixing rules for predicting the liquid density of pure refrigerants and liquefied natural gas.
- EoS Methods and Volume Translated
The equations of state are used in commercial simulation software for predicting phase behavior and thermodynamic properties. Generally, EoSs need a few parameters (usually two or three) that are normally obtained from critical properties. The cubic equations of state (EoS) give relatively accurate results for predicting vapor-liquid equilibria, especially for non-polar or slightly polar systems. Furthermore, these equations could be used to accurately predict vapor densities, enthalpy and entropy. These advantages encourage the researchers to augment EoS ability more than before, especially liquid density, although their accuracy for liquid density prediction is generally not as good as the correlations listed above. The popular EoSs such as SRK [7] and PR [8] predict liquid density with an average absolute error of about 8%, much higher than the correlations [9]. This large magnitude of error is not acceptable by industry; therefore they are not used for this purpose. In order to overcome this deficiency, a volume translated method has been developed by Peneloux et al. [10].
In this TOTM, we will present the method of volume translation for liquid density prediction and then demonstrate its application for liquid hydrocarbons such as pure methane, n-pentane, decane, pentadecane and carbon dioxide. In a future TOTM we will extend this procedure to multicomponent mixtures.
In order to improve the accuracy of EoSs for predicting liquid density, Peneloux et al. [10] proposed the following correction.
In the above equation,![]() is the corrected liquid specific volume,
is the corrected liquid specific volume, ![]() is the liquid specific volume calculated by SRK or PR EoS, MW is the molecular weight, ρL is the liquid density, and the correction term or volume shift factor “c” is determined from experimentally measured liquid density. It is normally regressed against several data points. In the absence of experimentally regressed value, it can be estimated as follows:
is the liquid specific volume calculated by SRK or PR EoS, MW is the molecular weight, ρL is the liquid density, and the correction term or volume shift factor “c” is determined from experimentally measured liquid density. It is normally regressed against several data points. In the absence of experimentally regressed value, it can be estimated as follows:
where ZRA, is the Rackett [10] parameter, R is the gas constant and TC and PC are the critical temperature and pressure, respectively.
In this work, we determined the value of “c” by minimizing the absolute error between experimentally measured liquid densities [11] and the corresponding predicted values using MathCad software. To achieve this, the following equation was defined.
where NP is the number of data points, ρexp is the experimental liquid density. The value of “c” was determined by minimizing f(c). In MathCad nomenclature the appropriate command is:
Results and Discussion:
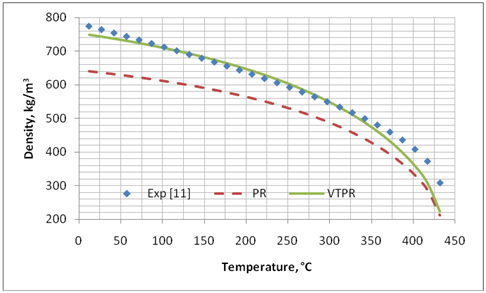
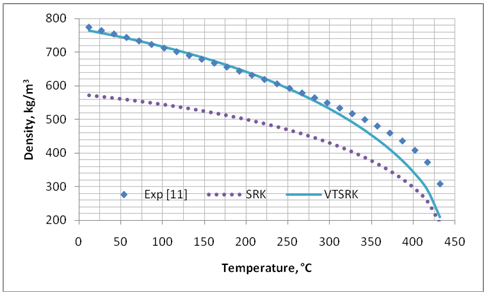
We applied the preceding procedure to several pure compounds shown in Table 1. The temperature and pressure ranges, and the optimized values of “c” for both PR and SRK EoS as well ZRA for the RSD correlation are shown in Table 1. Figures 1 and 2 present graphical comparisons between the predicted and experimental liquid density values of pentadecane. Similar trends were observed for the other compounds. Table 2 presents the summary of error analysis for different methods for the pure compounds shown in Table 1.
Table 1. Optimized volume translated parameter, c, for different compounds
| Component | Temperature Range, °C | Pressure Range, kPa | NP | SRK c x 106 m3/mol | SRK (c/b) | PR c x 106 m3/mol | PR (c/b) | ZRA |
| Methane | -182 to -90 | 12 to 3464 | 55 | -0.3506 | -0.012 | 4.059 | 0.151 | 0.2891 |
| n-Pentane | -121 to 124 | 0.0003 to 2548 | 21 | -11.36 | -0.113 | 2.04 | 0.023 | 0.2686 |
| Decane | -13 to 344 | 0.006 to 2097 | 18 | -41.44 | -0.197 | -13.99 | -0.074 | 0.2522 |
| Pentadecane | 12 to 432 | 0.0002 to 1421 | 29 | -92.89 | -0.270 | -48.25 | -0.156 | 0.2385 |
| CO2 | -56 to 18 | 531 to 5463 | 63 | -4.534 | -0.153 | 0.955 | 0.036 | 0.2719 |
Figure 1. Comparison of predicted liquid density of C15H32 by PR and volume translated PR (VTPR) against experimental data [11]
Figure 1. Comparison of predicted liquid density of C15H32 by SRK and volume translated SRK (VTSRK) against experimental data [11]
Table 2 indicates that considerable improvements are obtained by applying volume translated correction to liquid specific volume (or liquid density) predicted by PR and SRK. However, the accuracy of the COSTALD, RSD and NM correlations are still by far much better than the volume translation applied to these two EoSs. The accuracy of EoS and its volume translated correction deteriorate as the critical point is approached.
Table 2. Summary of error analysis for different methods studied
| Component | Average absolute Error % | ||||||
| PR | VTPR | SRK | VTSRK | RSD | NM | COSTALD | |
| Methane | 9.65 | 2.13 | 3.96 | 3.68 | 0.12 | 0.20 | 0.12 |
| n-Pentane | 2.64 | 2.14 | 11.03 | 3.07 | 0.14 | 0.15 | 0.11 |
| Decane | 7.62 | 3.09 | 18.16 | 4.43 | 1.04 | 0.70 | 0.95 |
| Pentadecane | 14.08 | 3.63 | 23.71 | 4.61 | 0.20 | 1.13 | 0.25 |
| Carbon Dioxide | 2.68 | 2.24 | 11.25 | 3.48 | 0.28 | 0.12 | 0.31 |
| Overall Average | 7.33 | 2.65 | 13.62 | 3.85 | 0.36 | 0.46 | 0.35 |
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses; G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing-Special).
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By Dr. Mahmood Moshfeghian
Reference:
- Campbell, J. M. “Gas conditioning and processing, Volume 1: Fundamentals,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.
- Engineering Data Book, 12th Editions, Gas Processors and Suppliers Association Data Book, Tulsa, Oklahoma, 2004.
- Hankinson, R. W., Thomson, G. H., AIChE J., Vol. 25, no. 4, pp. 653-663, 1979.
- Spancer, C. F., and Danner, R. P., J. Chem. Eng. Data, vol. 17, no. 2, pp. 236-241, 1972.
- Rackett, H. G., J. Chem. Eng. Data, vol. 15, No. 4, pp. 514-517, 1970.
- Nasrifar, Kh. and Moshfeghian, M., Fluid Phase equilibria Vol. 153, 231-242, 1998.
- Soave, G., Chem. Eng. Sci., Vol. 27, pp. 1197-1203, 1972.
- Peng, D. Y., and Robinson, D. B., Ind. Eng. Chem. Fundam., Vol. 15, p. 59, 1976.
- Nasrifar, Kh. and M. Moshfeghian, J. of Fluid Phase Equilibria, Vol. 158-160, pp. 437-445, 1998.
- Peneloux, A. E., Rauzy, E., and Freze, R., Fluid Phase Equilib., Vol. 8, pp. 7-23, 1982
- Vargaftik, N.B., Handbook of Physical Properties of Liquids and Gases (Pure Substances and Mixtures), 2nd ed., English Translation, Hemisphere Publication, 1975.

the published data are very interesting and I thank you for the article,
I would suggest to mention in the list also the extended Peng Robinson and Soave Redlich Kwong included in Prode Properties, Prode has specific parameters calculated for best fitting of vapor pressure, liquid density and latent heat of many pure compounds including hydrocarbons and polar fluids as water, glycols etc. that means that you get an average error of less than 2% for water or glycol liquid density and latent heats.
That is an important factor when simulating a gas dehydration plant.
Also Prode includes CPA PR and SRK, these are very accurate with associating fluids as water and glycol, I have tested accuracy for calculated values of water liquid density, average errors are about 1% from melting point temperature up to 0.95 * critical point.
I think this information could interest many engineers.