Accurate measurement and prediction of crude oil and natural gas liquid (NGL) products vapor pressure are important for safe storage and transportation, custody transfer, minimizing vaporization losses and environmental protection. Vapor pressure specifications are typically stated in Reid Vapor Pressure (RVP) or/and True Vapor Pressure (TVP). In addition to the standard procedures for their measurements, there are rigorous and shortcut methods for their estimation and conversion.
Based on ASTM D323, there are figures and monographs for conversion of RVP to TVP for NGLs (Natural Gas Liquids) and crude oil at a specified temperature [1, 2]. Continuing the February 2016 [3] Tip of The Month (TOTM), this tip will present simple correlations for determination TVP and RVPE (Reid Vapor Pressure Equivalent) as described by ASTM Standard D6377-14 [4] at a specified temperature. The correlations are easy to use for hand or spreadsheet calculations.
Standard D6377 describes the use of automated vapor pressure instruments to determine the vapor pressure exerted in the vacuum of crude oils. This test method is suitable for testing samples that exert a vapor pressure between 25 kPa and 180 kPa at 37.8 °C (3.63 psia and 26.1 psia at 100 °F) at vapor-liquid ratios from 4:1 to 0.02:1 (V/L = X = 4 to 0.02). A TVP reading can be determined by taking vapor pressure measurements at different expansion (V/L = X) ratios and extrapolating to V/L= X = 0. Refer to reference [4] for detail description of this standard procedure.
To demonstrate the ASTM Standard D6377 procedure we generated vapor pressures of a sample condensate shown in Table 1 at four expansion ratios of X = 1, 2, 3, 4 using ProMax simulation program [5] based on the Soave Redlich Kwong equation of state [6]. In this table, the heavy ends are presented by F-fractions and their properties are shown in Table 2.
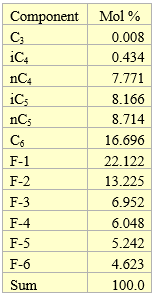
Table 1. Composite of condensate
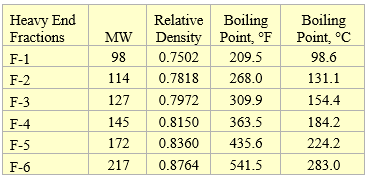
Table 2. Properties of the heavy end fractions used in Table 1
Table 3 presents the generated vapor pressure at 37.8 °C (100 °F) for four expansion ratios by ProMax mimicking the experimental measurements.
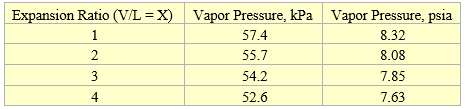
Table 3. Vapor pressure at 37.8 °C (100 °F) for four expansion ratios
Quadratic Equation:
The vapor pressures (VP) as a function of expansion ratio (X) in Table 3 were curve fitted to a quadratic equation as follows.
VP = a + bX +cX2
The fitted parameters a, b, and c are presented in Table 4 for pressures of Table 3 in kPa and psia.
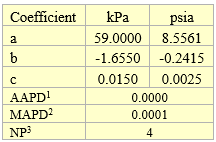
Table 4. Fitted parameters for quadratic equation
1AAPD = Average Absolute Percent Deviation
2MAPD = Maximum Absolute Percent Deviation
3NP = Number of data Points (NP)
Figure 1 presents the generated vapor pressure (filled circles) and the quadratic fit (solid line) of the condensate of Table 1. The extrapolated vapor pressure at X=0 (expansion ratio) is 8.56 psia (59.0 kPa). This extrapolated vapor pressure matches very closely with the predicted bubble point of condensate of Table 1 by ProMax.
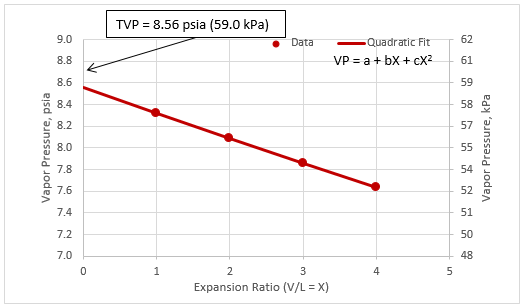
Figure 1. Quadratic fit of vapor pressure vs. expansion ratio
Exponential Equation:
The vapor pressures (VP) as a function of expansion ratio (V/L = X) in Table 3 can also be fitted to an exponential equation as follows.
VP = αe(βX)
The fitted parameters α and β are presented in Table 5 for pressures in Table 3 in kPa and psia.
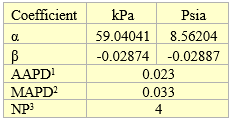
Table 5. Fitted parameters for exponential equation
1AAPD = Average Absolute Percent Deviation
2MAPD = Maximum Absolute Percent Deviation
3NP = Number of data Points (NP)
Figure 2 presents the generated vapor pressure (filled circles) and the exponential fit (solid line) of the condensate of Table 1. The extrapolated vapor pressure at X=0 (expansion ratio) is 8.56 psia (59.0 kPa). Similar to the quadratic fit, this extrapolated vapor pressure matches very closely with the predicted bubble point of condensate of Table 1 by ProMax.
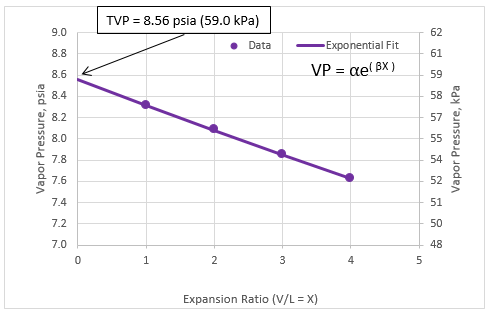
Figure 2. Exponential fit of vapor pressure vs. expansion ratio
ASTM D6377 RVPE:
The RVPE (Reid Vapor Pressure Equivalent) can be estimated by the following correlations:
a. Average bias of different crude oils [7]
RVPE = A x VPCRX=4 (at 100 °F or 37.8°C) + B
where A = 0.752 and B=0.88 psi (6.07 kPa).
For the condensate of Table 1 and from Table 3, VPCRX=4 = 7.63 psi (52.63 kPa)
RVPE = 0.752 x 7.63 + 0.88 = 6.62 psi
RVPE = 0.752 x 52.62 + 6.07 = 45.64 kPa
b. New correlation for ‘live’ crude oils (for samples in pressurized floating piston cylinders) [4]
RVPE = 0.834 x VPCRX=4 = 7.63 psi (52.63 kPa)
RVPE = 0.834 x 7.63 = 6.36 psi
RVPE = 0.834 x 52.62 = 43.89 psi
c. New correlation for ‘dead’ crude oils (for samples in non-pressurized 1-liter sample containers) [4]
RVPE = 0.915 x VPCRX=4 (at 100 °F or 37.8°C)
Summary:
For accurate measurements, standard procedures outlined in ASTM D6377–14 and other guidelines should be consulted. Several organizations are currently working to improve the accuracy of TVP estimation from RVP and/or VPCRx (ASTM D6377) measurement techniques. In all cases, Federal and State Laws and Regulations should be followed for safety and environmental protection.
A quadratic and an exponential correlation were presented to curve fit the measured vapor pressures at different expansion (V/L = X) ratios (e.g. 1, 2, 3, and 4). To demonstrate ASTM D6377 true vapor pressure measurements, a sample condensate vapor pressures at expansion ratios of V/L = X = 1, 2, 3, and 4 were estimated by ProMax, mimicking vapor pressure measurements. The estimated vapor pressures were curve fitted and extrapolated to zero expansion ratio (V/L = X) to estimate TVP. Then correlations of D6377 were used to estimate RVPE using the vapor pressure measurement at the expansion ratio of V/L = X = 4.
Figures 1 and 2 present almost a linear relationship between vapor pressure vs expansion ratio due to the narrow range of expansion ratio (1 through 4). As shown in the Appendix, for a wider range of expansion ratios (5 through 50), vapor pressure vs expansion ratio is non-linear. In addition, the quadratic fit with three coefficients gives a better fit compared to exponential fit with only two coefficients.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing), G5 (Advanced Applications in Gas Processing), and PF4 (Oil Production and Processing Facilities), courses.
PetroSkills offers consulting expertise on this subject and many others. For more information about these services, visit our website at http://petroskills.com/consulting, or email us at consulting@PetroSkills.com.
By: Dr. Mahmood Moshfeghian
References:
- Campbell, J.M., Gas Conditioning and Processing, Volume 1: The Basic Principles, 9th Edition, 2nd Printing, Editors Hubbard, R. and Snow–McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, 2014.
- ASTM D323: Standard Test Method for Vapor Pressure of Petroleum Products (Reid Method), 1999.
- Moshfeghian, M., http://www.jmcampbell.com/tip-of-the-month/2016/02/correlations-for-conversion-between-true-and-reid-vapor-pressures-tvp-and-rvp/, 2016
- ASTM D6377: Standard Test Method for Determination of Vapor Pressure of Crude Oil: VPCRX (Expansion Method), 2014
- ProMax 4.0, Bryan Research and Engineering, Inc., Bryan, Texas, 2017.
- Soave, G., Chem. Eng. Sci. Vol. 27, No. 6, p. 1197, 1972.
ASTM D6377: Standard Test Method for Determination of Vapor Pressure of Crude Oil: VPCRx (Expansion Method), 2003.
Appendix:
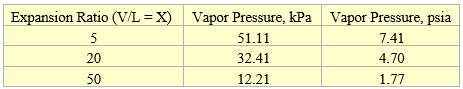
Table 3A. Vapor pressure at 37.8 °C (100 °F) for three expansion ratios
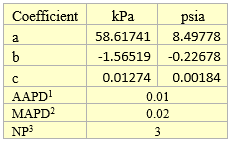
Table 4A. Fitted parameters for quadratic equation
1AAPD = Average Absolute Percent Deviation
2MAPD = Maximum Absolute Percent Deviation
3NP = Number of data Points (NP)
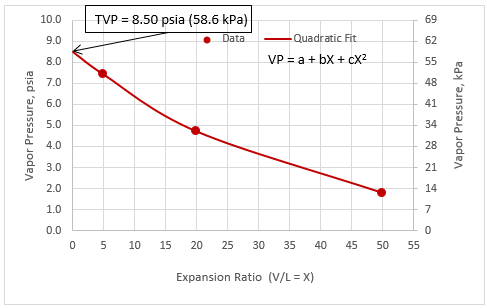
Figure 1A. Quadratic fit of vapor pressure vs. expansion ratio
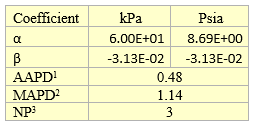
Table 5A. Fitted parameters for quadratic equation
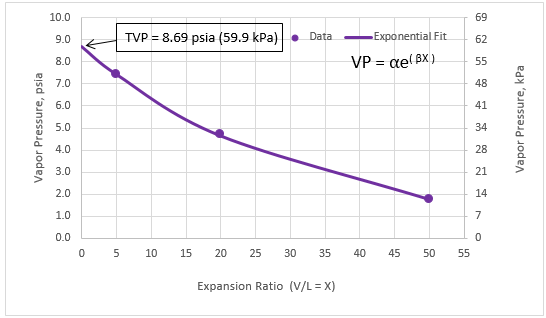
Figure 2A. Exponential fit of vapor pressure vs. expansion ratio

Good day,
Thanks for your informative articles.
I want to know when are you issuing December 2017.
Thanks and best regards,