In the last “Tip of the Month”, we evaluated the accuracy of two commercial process simulators against the experimental data. In this “Tip of the Month”, we will evaluate the accuracy of three shortcut methods for prediction of depression of hydrate formation temperature in the presence of two common inhibitors, methanol (MeOH) or mono ethylene glycol (MEG). We have used the same set of experimental data from the previous “Tip of the Month” as the basis for our evaluation. These shortcut methods may be used to calculate the required concentration of inhibitor and the injection rate for dew point correction process, NGL (Natural Gas Liquid) recovery, or pipeline transportation of natural gas. The detail of the calculation procedure is presented in chapter 6 of Volume 1 of Gas Conditioning and Processing [1]. The three methods evaluated are the Hammerschmidt (HA) [2], Nielsen- Bucklin (NB) [3], and Moshfeghian-Maddox (MM) [4]. These methods were used to predict hydrate formation temperature in the presence of inhibitors. Pure compounds as well as multi component natural gas mixtures covering a wide pressure range of up to 14500 psia (100 MPa) have been studied. Even though the shortcut methods presented in reference [1] could have been used, the required hydrate formation temperatures in the absence of inhibitor (pure water) were predicted by Parish and Prausnitz (PP) [5] for all three methods. This assured the same basis and accurate results. The strength and limitation of these methods are identified and recommendations for industrial applications have been made.
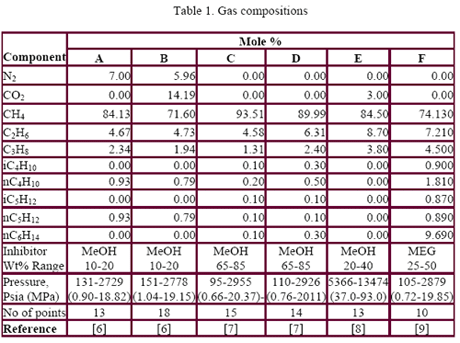
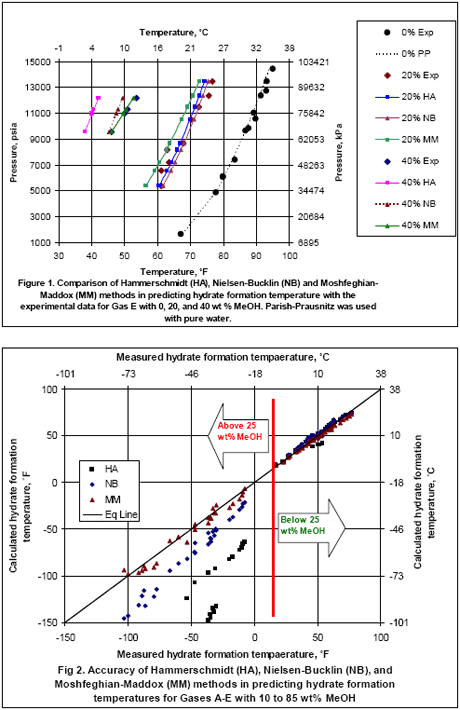
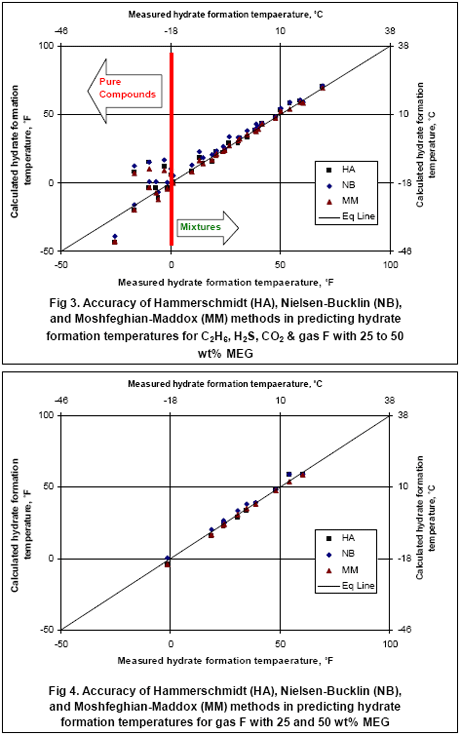
Table 1 presents the composition, inhibitor weight % range, pressure range, number of points, the reference of the experimental data for the gas mixtures studied in this work. The ability of the three methods to predict the hydrate formation temperature for gas E is shown in Figure 1. The accuracy of these methods, for CH4, C2H6, C3H8, CO2, H2S and their mixtures as shown in Table 1, are presented in Figures 1 and 2 for MeOH and Figures 3 and 4 for MEG.
Figure 1 indicates that the PP method predicts the hydrate formation temperature for gas E in the presence of pure water (0 wt% MeOH) accurately. This method was used to add its perdition temperature to the depression temperature predicted by the three shortcut methods for the sake of easy comparison with the experimental data. Figure 1 also indicates that all three methods give good results for 20 wt% MeOH; however, for 40 wt% MeOH, the HA results deviate from the experimental data considerably.
The analysis of Figures 2 indicates that all three methods give accurate results for temperature as low as 20 °F (-6.7 °C) equivalent to maximum of 25 wt% MeOH, but at lower temperature (or higher MeOH concentration) the HA method deviates from the experimental data considerably. For lower temperatures, the MM gives better results than the NB method.
Figure 3 and 4 indicate that all three methods give accurate results for gas mixture F up to concentration of 50 wt% MEG (as low as 0 °F or -18 °C) for which experimental data were available but their prediction for some of the pure compounds below this temperature is questionable.
To learn more about inhibitor injection we suggest participating in our G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special) courses.
By: Dr. Mahmood Moshfeghian
References:
- Campbell, J. M., “Gas Conditioning and Processing, Vol. 1, the Equipment Modules, 8th Ed., J. M. Campbell and Company, Norman, Oklahoma, 2001
- Hammerschmidt, E.G., ‘Formation of gas hydrates in natural gas transmission lines,” Ind & Eng. Chem, Vol. 26, p. 851, 1934
- Nielsen, R. B. and R.W. Bucklin, “Why not use methanol for hydrate control,” Hydrocarbon Processing, Vol 62, No. 4, P 71, April 1983
- Moshfeghian, M. and R. N. Maddox, “Method predicts hydrates for high- pressure gas streams,” Oil and Gas J., August 1993.
- Parrish, W. R. and J. M. Prausnitz. “Dissociation Pressures of Gas Hydrates Formed by Gas Mixtures”, Ind. Eng. Chem. Proc. Dev.,11, 1, 26-35, (1972).
- Ng, Heng-Joo, and D.B. Robinson, Research Report RR-66, Gas Processors association, Tulsa, Oklahoma, 1983
- Ng, Heng-Joo, C.J. Chen, and D.B. Robinson, research report RR-106, Gas Processors Association, Tulsa, Oklahoma, 1987
- Blanc, C., and Tournier-Lasserve, J., World Oil, November, 1990.
- Ng, Heng-Joo, C.J. Chen, and D.B. Robinson, research report RR-92, Gas Processors Association, Tulsa, Oklahoma, 1985

Dear Dr.Moshfeghian,
Hammerschmidt and Neilson corrolations, are used to estimate the required mass flow rate of hydrate inhibitor (such as MeOH, EG) based on required depression of hydrate formation temperature in gas phase, as liquid phase contain ONLY water and inhibitor components. For example, Fig 20-45 is used to convert methanol mole fraction to mass fraction in two-component liquid system. And in Eq. 20-4, only terms of X (MeOH wt%) and 1-X (water wt%) are observed, that means only two component assumed in liquid phase. In line with this understanding, at chilling system (EG) that liquid phase encompasses water, inhibitor, and hydrocarbon component, the credit of use of mentioned equations in chilling system will be questionable.
I appreciate you kindly advise/confirm my understanding.
Kind regards,
Hooman Tabaraei
[url=http://www.okakaku.com/brand-1-copy-7-cheap-0-max0-attr0.html]これはsevenfridayシリーズで私の問題である。この音はセンセーショナルかもしれないとして、私はシリーズの古典であったと思っています。それはそれとして崇拝して非常に軽蔑されるかもしれません、しかし、それに耐えた。その価格の点で、そのように見えるのは、それがその生産実行上の数千と何千もの特徴のコレクションに設定されている。その影から踏み出すとき、単に、sevenfriday sevenfridayシリーズその先駆者に接続するために何かをしました。彼らが、何sevenfriday 01シリーズv 3で明らかなように、同一のパターンレイアウトを次のリリース計画、シェアードソースからインスピレーションをとって、各シリーズの各モデルを用いた ガガミラノスーパーコピー 。で、シリーズは、1、2、3の各々と、それぞれに触発された産業の本質によって、産業革命、産業用エンジン。これは、消費者は各々の以降のシリーズが好きになったのですdnaのいくつかの表現かもしれないが、多くのバリエーションが期待できる手段(または憎しみ)は、以前のリリース。それが意味するもののために人々と女の子は、sevenfridayプレスリリース文書で請求されたラインの下の宣伝の年を維持するのにかなり創造的になるだろうということです。[/url]
Absolutely written articles, Really enjoyed examining.