Many materials may be added to water to depress both hydrate and freezing temperatures. For many practical reasons an alcohol or one of the glycols is injected as an inhibitor, usually methanol, diethylene glycol (DEG) or mono ethylene glycol (MEG). All may be recovered and recirculated, but economic of methanol recovery may not be favorable in many cases. Total injection rate is that needed to provide the necessary inhibitor concentration in the liquid water plus that inhibitor which enters the vapor and hydrocarbon liquid phases. Any inhibitor in the vapor phase or liquid hydrocarbon phase has little effect on hydrate formation conditions. Determination of the amount and concentration of inhibitors and their distribution in different phases are very important for practical purposes and industrial applications. Therefore, in order to determine the required amount and concentration of these inhibitors, several thermodynamic models for hand and rigorous calculations have been developed and incorporated into the computer software.
In this Tip of the Month, we will evaluate the accuracy of two commercial process simulators “A” and “B” against the experimental data. These softwares were used to predict hydrate formation condition in the presence of inhibitor. Pure gas component as well as multi component natural gas mixtures covering a wide pressure range of up to 100 MPa have been studied. These softwares may be used to simulate an integrated NGL (natural gas liquid) plant with inhibitor injection and regeneration process. The optimum concentration and circulation rate are determined using different softwares. The strength and limitation of these softwares are identified and recommendations for industrial applications have been made.
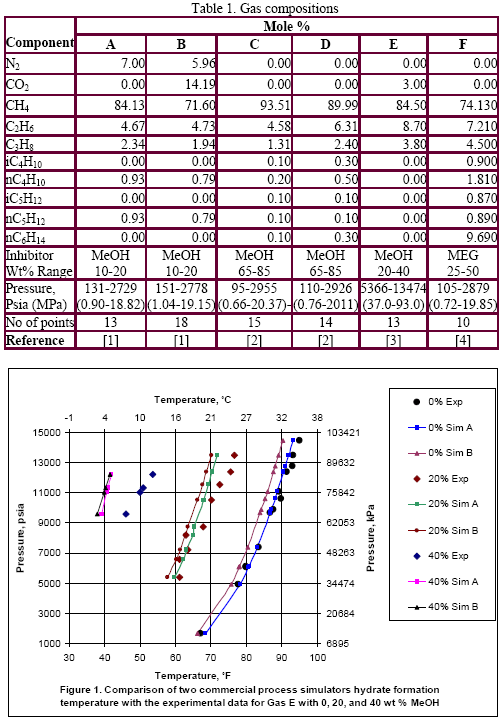
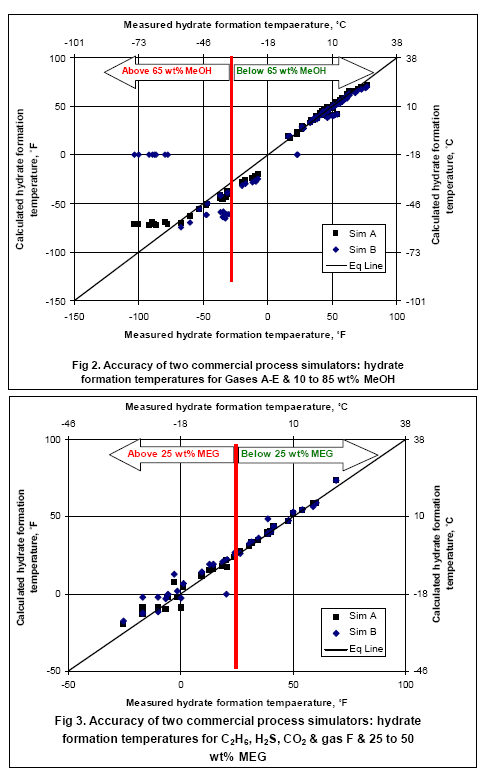
Table 1 presents the composition, inhibitor weight % range, pressure range, number of points, the reference of the experimental data for the gas mixtures studied in this work. The ability of the two softwares to predict the hydrate formation temperature for gas E is shown in Figure 1. The accuracy of these softwares for CH4, C2H6, C3H8, CO2, H2S and their mixtures as shown in Table 1 are presented in Figures 2 and 3; for methanol and MEG, respectively
The analysis of Figures 2 and 3 indicates that for methanol inhibition, the lower limits of hydrate formation temperatures are -75°F (-60°C) for Sim A (maximum of 70 wt% methanol) and -25°F (-32°C) for Sim B (Maximum of 50 wt% methanol). It should be pointed out that Sim B could not converge for cases of 85 wt% methanol. For MEG, both softwares give accurate results down to 25°F (-4°C) corresponding to 25 wt% MEG; however, for lower temperatures the accuracy drops while the Sim A gives better results. To learn more about inhibitor injection we suggest participating in our G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special) courses.
By: Dr. Mahmood Moshfeghian
References:
- Ng, Heng-Joo, and D.B. Robinson, Research Report RR-66, Gas Processors association, Tulsa, Oklahoma, 1983
- Ng, Heng-Joo, C.J. Chen, and D.B. Robinson, research report RR-106, Gas Processors association, Tulsa, Oklahoma, 1987
- Blanc, C., and Tournier-Lasserve, J., World Oil, November, 1990.
- Ng, Heng-Joo, C.J. Chen, and D.B. Robinson, research report RR-92, Gas Processors association, Tulsa, Oklahoma, 1985

Great stuff!
I enjoy you because of your own efforts on this site. Ellie take interest in working on investigation and it is easy to understand why. Most of us know all relating to the dynamic means you deliver precious guides via your blog and as well invigorate contribution from people on this idea and our princess is always learning so much. Take pleasure in the rest of the new year. You’re conducting a powerful job.