Liquid density is needed for process simulation and equipment design. For example, accurate predictions of liquid density are needed for calculation of pressure drop in a piping/pipeline and vessel sizing. Accurate liquid density is also essential for custody transfer.
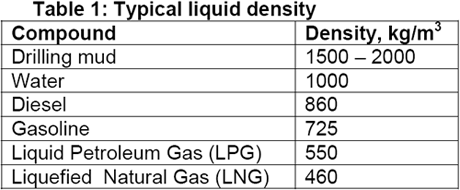
Liquid density ranges from a few hundred above thousand to couple of 100 kg/m3. Table 1 presents typical range of liquid densities where as typical re-injection gas has a density in the range of 125 to 150 kg/m3 and pipeline gases at 7000kPa has a density in the order of 70 kg/m3.
Liquid densities are sometime expressed in terms of relative density (specific gravity) or API gravity. The relative density,? , is defined as:
and the API (American Petroleum Index) gravity is:
Depending on the applications, three different methods can be used to compute liquid density in addition to direct laboratory measurement. These methods are (a) charts and monographs, (b) correlations and (c) EoS or volume translated EoS, of which the correlations are usually the most accurate.
For the LNG density measurement and calculation, one of the standard procedures practiced in industry is ISO 6578: 1991 [1]. This procedure specifies the calculations to be made to adjust the volume of a liquid from the conditions at measurement to the equivalent volume of liquid or vapor at a standard temperature and pressure, or to the equivalent mass or energy (calorific content). Annexes A to H of this procedure form an integral part of this standard.
Generalized Charts
There are several generalized charts for prediction of liquid density of petroleum fluids and hydrocarbons [2].
The relative density of petroleum fluids are normally expressed in terms of two of three characteristics- API gravity at 15°C, the Watson characterization factor, KW, or the mean average boiling point. The Watson characterization factor is defined in terms of mean average boiling point, Tb, and the relative density at standard condition.
The charts normally present the relative density of paraffinic hydrocarbon mixtures at their boiling point or bubble point temperature and pressure. These charts apply to mixtures as well to pure components. Alignment points for paraffinic hydrocarbon mixtures and pure components are located according to their molecular weight. The accuracy of these charts is generally within 3 % of the measured values. However, the accuracy is somewhat less for mixtures having molecular weights less than 30 where temperature is low, and where the methane content is high or reduced temperatures above 0.9 [3].
EoS Methods and Volume Translation
The EoSs are used in commercial simulation softwares for predicting phase behavior and thermodynamic properties. Generally, EoSs need a few parameters (usually two or three) that are normally obtained from critical properties. The cubic equations of state (EOS) give accurate results for prediction of vapor-liquid equilibria, especially for non-polar or slightly polar systems. Furthermore, these equations could be used to accurately predict vapor densities, enthalpy and entropy. These advantages encourage the researchers to augment EoS ability more than before, especially, liquid density, although their accuracy for liquid density prediction is not as good as correlations. The popular EoSs such as SRK [4] and PR [5] predict liquid density with an average absolute error about 8%, much more than the correlations [6]. This large magnitude of error is not acceptable by industry; therefore; they are not used for this purpose. In order to overcome this deficiency, a volume translated method has been developed by Peneloux et al. [7]. The working equations are:
In the above equation, vSRK is calculated by SRK EoS and the correction term “c” as follows:
Correlations
In order to calculate liquid density reliably, several correlations such as COrresponding STAte Liquid Density (COSTALD) and modified Rackett equation by Spencer and Danner (RSD), have been developed.
COSTALD correlation: The COSTALD correlation, Hankinson and Thomson [8], requires two parameters, ?SRK, the optimized value of the acentric factor based on the SRK EoS and V*, the pure component characteristic volume.
The RSD correlation: Spencer and Danner [9] improved the liquid density correlation of Rackett [10]. The improved correlation for saturated liquid density calculation requires only ZRA, the improved compressibility factor.
In next “Tip of the Month” we will provide guidelines for use of these methods and present the results of a comparison study between these methods.
To learn more about liquid density and its impact on facilities calculation, design and surveillance, refer to JMC books and enroll in our G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special) courses.
By Dr. Mahmood Moshfeghian
Reference:
- http://www.iso.org
- Campbell, J. M. “Gas conditioning and processing, Volume 1: Fundamentals,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.
- Engineering Data Book, 10th and 11th Editions, Gas Processors and Suppliers Association Data Book, Tulsa, Oklahoma, 1998.
- Soave, G., Chem. Eng. Sci., vol. 27, pp. 1197-1203, 1972.
- Peng, D. Y., and Robinson, D. B., Ind. Eng. Chem. Fundam., vol. 15, p. 59, 1976.
- Nasrifar, Kh. and M. Moshfeghian, J. of Fluid Phase Equilibria, 158-160, pp. 437-445, 1998.
- Peneloux, A. E., Rauzy, E., and Freze, R., Fluid Phase Equilib., vol. 8, pp. 7-23, 1982
- Hankinson, R. W., Thomson, G. H., AIChE J., vol. 25, no. 4, pp. 653-663, 1979.
- Spancer, C. F., and Danner, R. P., J. Chem. Eng. Data, vol. 17, no. 2, pp. 236-241, 1972.
- Rackett, H. G., J. Chem. Eng. Data, vol. 15, no. 4, pp. 514-517, 1970.

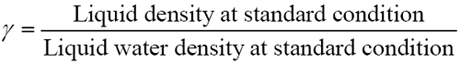
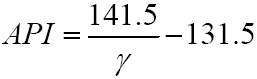
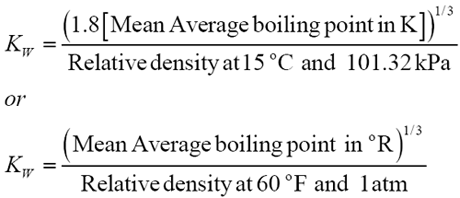
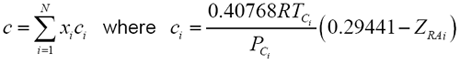
Howdy very nice blog!! Guy .. Excellent .. Amazing .. I’ll bookmark your blog and take the feeds additionally…I’m glad to seek out a lot of useful info right here in the post, we need develop more strategies in this regard, thank you for sharing.