In the last Tips of the Month, we discussed the phase behavior of water-sweet natural gas and water-sour natural gas mixtures. In this tip, we will demonstrate the acid gas–water phase behavior.
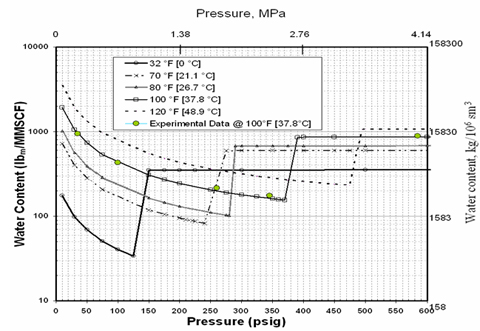
The water content of a gas depends on the system temperature, pressure and composition of the gas. The phase equilibria in the system H2S + water and CO2 + water are key to the discussion of the water content of an acid gas system. Figure 1 presents the water content of hydrogen sulfide predicted by ProMax [1] as a function of pressure and temperature. A limited number of experimental data points at 100°F [37.8°C] by Gillespie and Wilson [2] are also shown on this diagram. The behavior shown on this plot is quite complicated and explained by Carroll [3] thoroughly: “At low pressure the hydrogen sulfide + water mixture is in the gas phase. At low pressure the water content tends to decrease with increasing pressure, which is as expected. Eventually a pressure is reached where the H2S is liquefied. On this plot this is represented by the discontinuity in the curve and a broken line joins the phase transition. There is a step change in the water content when there is a transition from vapor to liquid. In the case of hydrogen sulfide the water content of the H2S liquid is greater than the coexisting vapor. This is contrary to the behavior for light hydrocarbons where the water content in the hydrocarbon liquid is less than the coexisting vapor.”
Figure 1. Water content of pure H2S predicted by ProMax, experimental data [2]
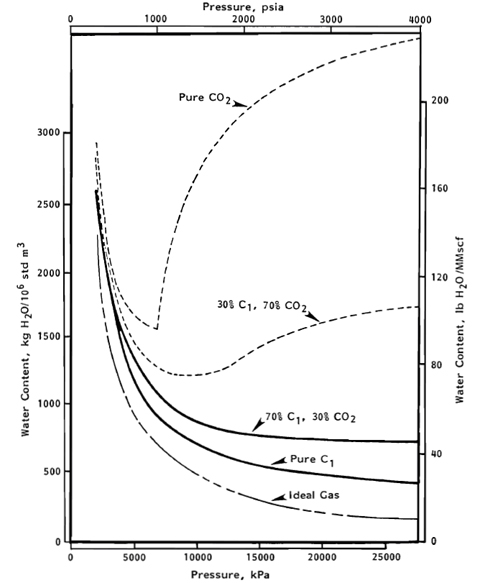
In general the phase behavior of the system CO2 + water is as complex as that of the H2S + water system. The CO2-rich liquid phase only occurs for temperatures less than about 90°F [32.2°C]. As shown in Figure 2 reported by Maddox and Lilly [4], the water content of CO2 exhibits a minimum.
Figure 2. Predicted saturated water contents at 100°F [38°C] for CO2, CH4 and mixture of both [4]
There are several methods available that can be used to predict the water content of acid gases. All of these methods are based on equation of state and rigorous thermodynamic models. As described above, the phase behavior is complicated and extra care should be taken to assure a correct prediction. In the remaining section of this tip, we will demonstrate the capabilities of some of these methods.
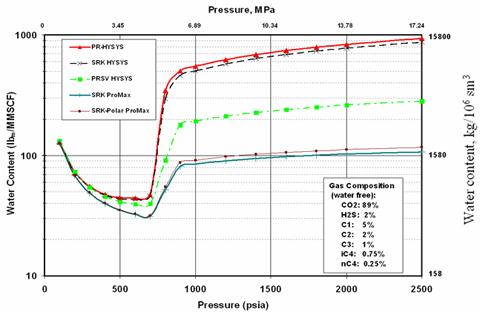
Figure 3 compares the water content calculation results for an acid gas stream by several methods in HYSYS [5] and ProMax [1]. The composition of the acid gas stream is shown in the inset of diagram. Even though at low pressures, all methods give close results, as can be seen from this figure, there are large differences at higher pressures.
Figure 3. Comparison of water content prediction by different methods at 59 °F [15°C]
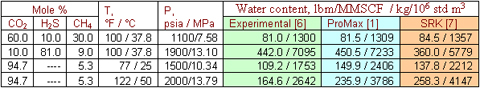
Table 1 gives another comparison of available methods for prediction of acid gas water content.
Table 1. Comparison of ProMax and modified SRK EOS results with the experimental water content [6] of several acid gas mixtures:
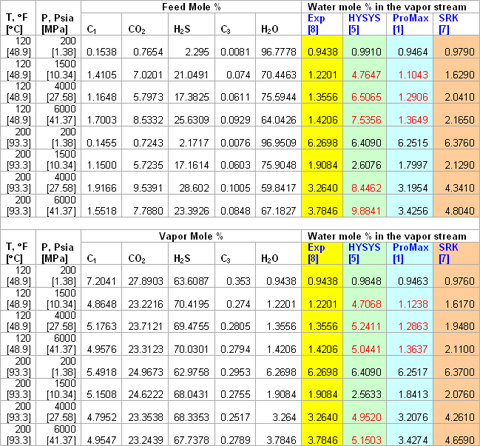
The experimental composition and predicted water content by HYSYS, ProMax and the modified SRK for eight acid gas mixtures are presented in Table 2. The upper part of this table reports the measured mole percent of the feed stream and the lower part shows the experimental vapor stream compositions in mole percent. Based on the feed compositions, three-phase flash calculations were performed and the resulting vapor stream water content (mole %) are shown in the last three columns (upper part).
For each vapor stream, the saturated water content was predicted by the above methods and is presented in the lower portion of this table. As can be seen from this table, ProMax predict saturated water content reasonably well. The red figures in Table 2 indicate that the methods predict a non-aqueous liquid phase instead of the vapor phase. Based on a dry basis phase envelope, the conditions for these mixtures were dense phase/compressed liquid.
Table 2. Conditions and compositions of 8 acid gases and their saturated water contents
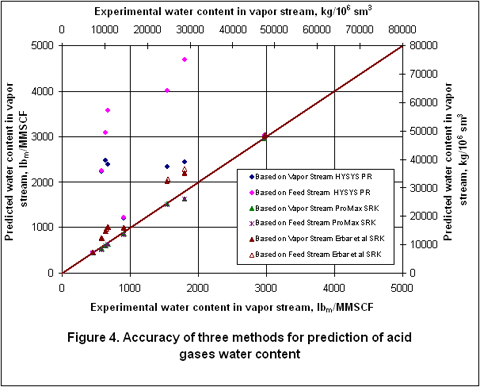
Figure 4 also compares the accuracy of the above methods graphically. This figure clearly indicates that ProMax gives the most accurate results. The Erbar et al. [7] SRK method also gives reasonable results.
To learn more about similar cases and how to minimize operational problems, we suggest attending
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing), G5 (Gas Conditioning and Processing – Special), G-6 Gas Treating and Sulfur Recovery and RF-61 Refinery Gas Treating, Sour Water, Sulfur and Tail Gas courses.
By Wes Wright and M .Moshfeghian
Reference:
- ProMax 2.0, Bryan Research and Engineering, Inc., Bryan, Texas, U.S.A., 2007
- Gillespie, P.C. and G.M. Wilson, “Vapor-Liquid Equilibrium Data on Water-Substitute Gas Components: N2-H2O, H2-H2O, CO-H2O, H2-CO-H2O, and H2S-H2O” Research Report RR-41, GPA, Tulsa, OK, 1980.
- Carroll, J.J., “The water content of acid gas and sour gas from 100 to 220 °F and pressures to 10,000 Psia,” Presented at the 81st Annual GPA Convention, Dallas, Texas, USA, March 11-13, 2002.
- Maddox, R.N., L.L. Lilly, “Gas conditioning and Processing, Vol 3: Computer Applications and Production/Processing Facilities”, John M. Campbell & Company, Norman, USA, 1982.
- ASPENone, Engineering Suite, HYSYS Version 2006, Aspen Technology, Inc., Cambridge, Massachusetts U.S.A., 2006.
- Huang, S.S.-S., A.-D. Leu, H.-J. Ng, and D.B. Robinson, “The Phase Behavior of Two Mixtures of Methane, Carbon Dioxide, Hydrogen Sulfide, and Water” Fluid Phase Equil. 19, 21-32, 1985.
- Erbar, J.H., A.K. Jagota, S. Muthswamy, and M. Moshfeghian, “Predicting Synthetic Gas and Natural Gas Thermodynamic Properties Using a Modified Soave Redlich-Kwong Equation of State,” Gas Processor Research Report, GPA RR-42, Tulsa, USA, 1980.
- Ng, H.-J., C.-J. Chen, and H. Schroeder, “Water Content of Natural Gas Systems Containing Acid Gas”,Research Report RR-174, Gas Processors Association, Tulsa, OK, 2001.

[…] W. and M. Moshfeghian, “Acid Gas-Water Phase Behavior,” http://www.jmcampbell.com/tip-of-the-month/2007/12/acid-gas-water-phase-behavior/, December, […]
[…] November 2007 TOTM [2], and December 2007 TOTM [3], respectively. In this TOTM, we will revisit the acid gas-water phase behavior system. Specifically, different methods of predicting water content of acid gas systems are […]
I wanted to compose you one very small observation to be able to say thanks a lot again for these beautiful information you’ve provided above. It was quite remarkably generous of people like you to present unhampered all a lot of people would’ve advertised for an e book to earn some bucks on their own, most notably given that you could have tried it if you ever desired. Those ideas also served to become easy way to be certain that other individuals have the same dream like my personal own to know the truth more and more when it comes to this problem. I am sure there are thousands of more pleasant moments up front for individuals who check out your blog post.
Hi, i believe that i saw you visited my website
thus i got here to return the prefer?.I am attempting to find things to enhance my website!I guess its good enough to make use of
some of your concepts!!
My web site bing.com