Gas density is needed for process simulation and equipment design. For example, accurate predictions of gas density are needed for calculation of pressure drop in piping/pipeline and for vessel sizing. Accurate gas density is also essential for custody transfer metering. Gas density, ![]() , is calculated by:
, is calculated by:
Where:
&Gas density, kg/m3 (lbm/ft3)
Absolute temperature, K (ºR)
Pressure, kPa (psia)
MW Molecular weight kg/kmole (lbm/lbmole)
Gas compressibility factor
Universal gas constant, 8.314 (kPa)(m3)/(kmole)(K) or 10.73 (psia)(ft3)/(lbmole)(ºR)
In equation 1, “z” represents gas compressibility factor. For ideal gases, “z” is equal to 1. Gas densities are sometime expressed in terms of relative density (specific gravity), ![]() , and is defined as:
, and is defined as:
Substituting Equation 1 for gas and air into Equation 2 and assuming ideal gas behavior at standard conditions, Equation 2 will be transformed to:
At the standard condition and for simplicity, Equation 3 can be written as
In Equation 1, the key parameter is the compressibility factor “z”, which is a function of pressure, temperature and gas composition. Compressibility factor is a dimensionless surrogate of non-ideal gas density. In general, equations of state are probably the most widely used for calculation of z. They are not necessarily the most accurate. Empirical correlations developed for a specific mixture or a narrow range of mixtures provide better accuracy, but may be less general. An example would be the Katz chart which is quite good when applied to “sweet” pipeline quality gases, but less reliable for gases containing H2S, CO2 and/or N2. Figure 3.2 in Chapter 3 of Gas Conditioning and Processing [1] shows the Katz chart for sweet natural gases as prepared by Standing and Katz [2]. The chart was developed by using experimental data on methane binary mixtures with ethane, propane, butane and other natural gases over a wide range of composition with a maximum molecular weight of 40.
For fiscal metering of natural gas, an accurate experimental database has been developed and compressibility factor correlations, with uncertainties generally within ±0.2%, have been published in the industry standards, AGA Report No. 8 and ISO 12213. A summary of some common “z” correlations and their effect on gas measurement accuracy can be found in reference [3]. Since many people use the Katz compressibility factor chart, the question is often asked how it may be extended to gases containing H2S and CO2. There are two methods available for this application.
- The approach proposed by Robinson et al. [4]
- The approach proposed by Wichert and Aziz [5]
In this Tip of the Month (TOTM) we will demonstrate the accuracy of the second approach. The details of this method are presented in Chapter 3 of Gas Conditioning and Processing [1].
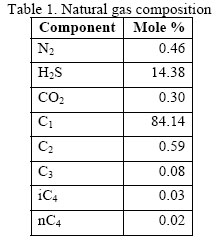
Let’s consider the gas mixture shown in Table 1 with total acid gas (H2S and CO2) of 14.68 mole percent. At 13.94 MPa (2021 psia) and 58 ºC (136 ºF), the compressibility factors are 0.797 (120.1 kg/m3) and 0.832 (114.8 kg/m3), using Katz chart and Wichert-Aziz method respectively. The percent deviation between two answers from each other is 4.4%.
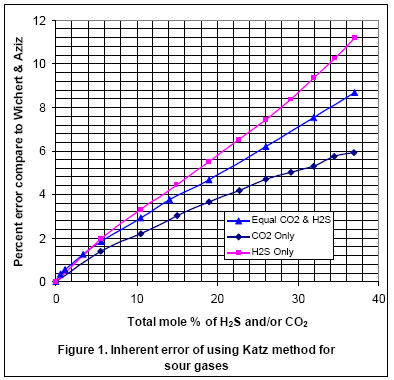
In order to show the effect of acid gas on compressibility factor determined from Katz chart and Wichert-Aziz methods, we varied the acid gas content of the gas in Table 1 from 0 to 37 mole percent. This was accomplished by diluting the non-acid gas components with a 50:50 mixture of CO2 and H2S. Figure 1 presents the percentage difference between the two methods as a function of acid gas content. The graph shows that as the H2S and CO2 content increases, the deviation of Katz chart from Wichert-Aziz method increases almost linearly. This graph also indicates that the percentage difference between the two methods is greater for the case of diluting gas with only H2S than only CO2.
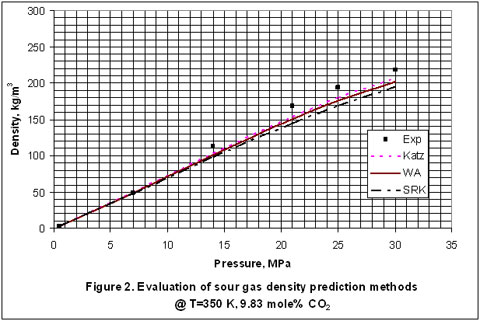
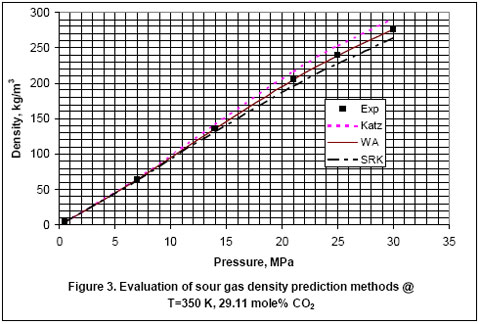
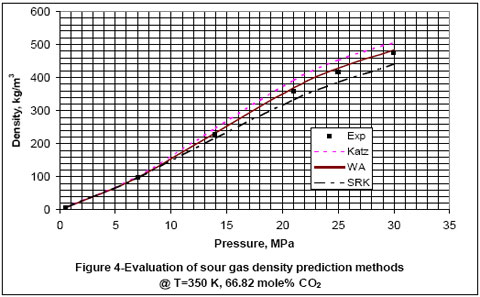
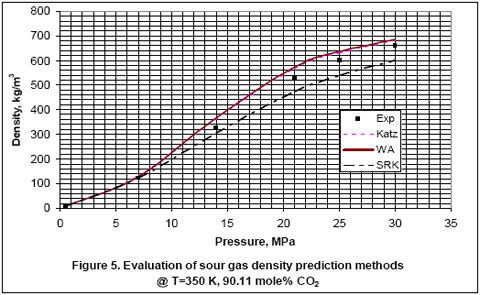
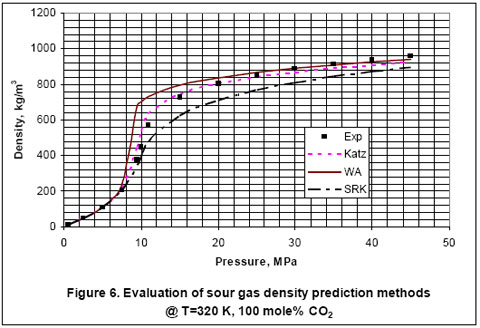
Next, we used the experimental data reported in the GPA RR-138 [6] and GPA RR 68 [7] to evaluate the accuracy of Katz, Wichert-Aziz and SRK equation of state (EOS) for binary mixtures of CO2 and CH4. The results of this evaluation are shown in Figures 2 through 6, for CO2 content of 9.83 to 100 mole percent. The figures indicate that the Katz correlation accuracy decreases as the mole percent of CO2 increases. However; Figure 5 indicates that as the gas becomes very rich in CO2, the accuracy of the Katz correlation and the Wichert-Aziz method are practically identical. Figure 6 shows that the Katz correlation best predicts the density of pure CO2, and also when the gas approaches pure CH4. The experimental data for pure CO2 in Figure 6 is from GPA RR 68 [7]. Figure 2 through 6 also indicate that the SRK EOS has low accuracy. In this study, a binary interaction parameter of 0.12 between CH4 and CO2 which had been determined from experimental vapor-liquid-equilibrium (VLE) data was used.
Based on the work done in this study, the following can be concluded:
- Katz correlation gives accurate results for pipeline quality gases (lean sweet gases)
- For pure CO2, Katz correlation is the most accurate in comparison to Wichert-Aziz method or the SRK EOS.
- For binary mixture of CH4 and CO2, Wichert-Aziz method gives the most accurate result for CO2 content of between 10 and 90 mole percent.
- As H2S and CO2 content increases, the accuracy of the Katz correlation decreases, but its accuracy increases as the mixture approaches a single (pure) component.
- The percentage difference between the Katz and Wichert-Aziz methods for gas mixtures containing acid gases is greater for H2S than CO2.
- Binary interaction parameters which have been optimized to predict VLE behavior, may not provide the best density prediction.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing), G5 (Gas Conditioning and Processing – Special), G6 (Gas Treating and Sulfur Recovery), RF61 (RefineryGas Treating, Sour Water, Sulfur and Tail Gas), PF-81 (CO2 Surface Facilities), and G40 (Process/Facility Fundamentals) courses.
By: Dr. Mahmood Moshfeghian
References:
- Campbell, J. M., and Hubbard, R. A., Gas Conditioning and Processing, Vol. 1 (8th Edition, 2nd Printing), Campbell Petroleum Series, Norman, Oklahoma, (2001).
- Standing, M.B. and Katz, D.L.; “Density of Natural gas gases,” AIME Trans., 146, 140-49 (1942)
- Hannisdal, N.E., “Gas Compression Equations Evaluated,” Oil and Gas J., p. 38-41 (May 4, 1987)
- Robinson, D. F. et al. Trans. AIME, Vol 219, P. 54, (1960).
- Wichert, E. and Aziz, K., Hydr. Proc., p. 119 (May 1972).
- Hwang, C-A., Duarte-Garza, H., Eubank, P. T., Holste, J. C. Hall, K. R., Gammon, B. E., March, K. N., “Thermodynamic Properties of CO2 + CH4 Mixtures,” GPA RR-138, Gas Processors Association, Tulsa, OK, June 1995
- Hall, K. R., Eubank, P. T., Holste, J., Marsh, K.N., “Properties of C02-Rich Mixtures Literature Search and Pure C02 Data, Phase I,” GPA RR-68, A Joint Research Report by Gas Processor Association and the Gas Research Institute, Gas Processors Association, Tulsa, OK, June 1985

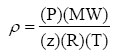
ちょうど約すべての小さなクラッチが鉄。また示したグッチ、中程度黒クラッチと彼らロックンロール、すべての黒衣装。黒のクラッチを特集アンティークグッチタッセルとソフト豪華な家庭用革。
[…] M., “How good are the shortcut methods for sour gas density calculations?,” PetroSkills tip of the month, Sep […]