Gas density estimates are of fundamental importance for process simulation, equipment design, and process safety engineering. In the previous Tip of the Month (TOTM), two shortcut methods for predicting sour and acid gas density were evaluated. We showed that Katz correlation gives accurate results for lean sweet gases and it is the most accurate in comparison to Wichert-Aziz method or the SRK EOS. For binary mixtures of CH4 and CO2, Wichert-Aziz method gives the most accurate result for CO2 content of between 10 and 90 mole percent. As H2S and CO2 content increased, the accuracy of the Katz correlation decreased, but its accuracy increased as the mixture approached a single component. The percentage difference between the Katz and Wichert-Aziz [1] methods for gas mixtures containing acid gases was greater for H2S than CO2.
Process simulation software often use the Benedict-Webb-Rubin-Starling (BWRS), Soave-Redlich-Kwong (SRK) and/or Peng-Robinson (PR) equations of state for gas density calculations. Other sources of gas density calculation are NIST REFPROP (Reference Fluid Thermodynamic and Transport Properties) program and GERG-2004 [2, 3], a reference equation of state for natural gases.
Due to the importance of CO2 injection for enhanced oil recovery and the increasing interest in CO2 capture and sequestration, this study was undertaken to evaluate the accuracy of density calculations for gases containing nil to 100% CO2. An experimental data base was used for the basis of comparison. The study reviews all of the above mentioned methods and will report their accuracies. Table 1 presents the summary of the temperature, pressure, and CO2 mole percent ranges for the data used in this study. The sources of experimental data were reference [4, 5]. This table also presents the average absolute average percent error and the overall average percent error.
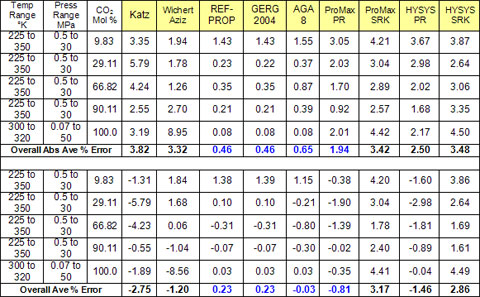
Table1 – Summary of error analysis and comparison of accuracy of sour gas and acid gas density prediction by several methods:
Table 1 provides the overall accuracy of the various methods. It should be noted that the relative accuracy of each method varies depending on the CO2-CH4 proportion, the temperature and pressure. The AGA 8 did not return values for many of the low temperature cases where two phases were present. These points were ignored in the analysis.
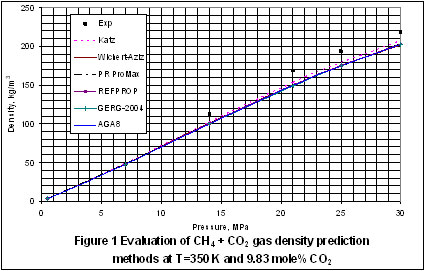
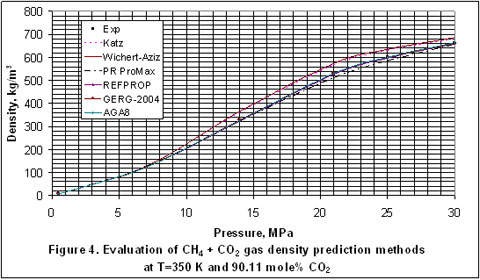
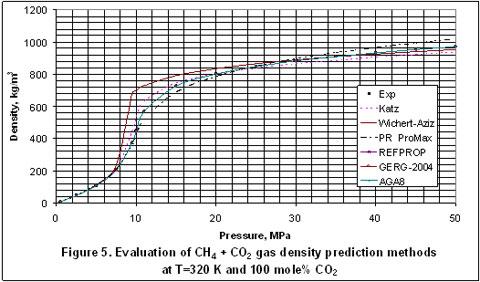
Next, we plotted the experimental data reported in the GPA RR-138 [3] and GPA RR 68 [4] to evaluate the accuracy of Katz, Wichert-Aziz and the best four of the detailed methods. The results of this evaluation for the T=350°K and 320°K cases are shown in Figures 1 through 5, for CO2 content of 9.83 to 100 mole percent. In Figure 1, Katz method is the most accurate and the accuracy of the other methods are almost the same.
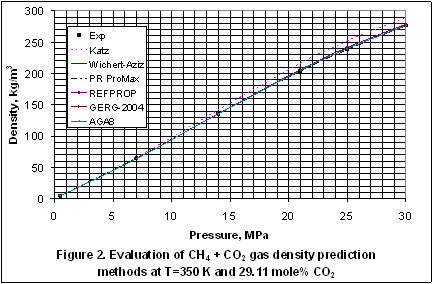
In Figure 2, Katz method has the least accuracy and even though the accuracy of the other methods look the same, GERG 2004 is slightly better than the others.
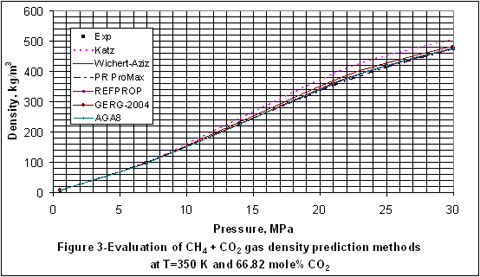
In Figure 3, Katz method again has the least accuracy and even though the accuracy of the other methods look the same, AGA8 provided slightly better estimates than the others.
In Figure 4, Wichert-Aziz method has the least accuracy and even though the accuracies of the other methods look the same, AGA8, GERG-2004 and REFPROP are slightly more accurate than the PR EOS.
In Figure 5, Wichert-Aziz method has the least accuracy and REFPROP, GERG 2004 and AGA 8 equally have the best accuracy.
Based on the work done in this study and in the previous TOTM, the following can be concluded:
- Katz correlation gives accurate results for pipeline quality gases (lean sweet gases)
- For pure CO2, AGA 8, REFPROP, and GERG 2004 methods equally are the most accurate method
- For binary mixtures of CH4 and CO2, REFPROP and GERG 2004 methods equally give the most accurate result for CO2 content of between 10 and 90 mole percent.
- As CO2 content increases, the accuracy of the Katz correlation decreases, but its accuracy increases as the mixture approaches a single (pure) component.
- The Peng-Robinson EOS provides a better density estimate than the SRK EOS.
- Results from either the PR or the SRK EOS in ProMax are slightly more accurate than the comparable results from HYSYS.
- Binary interaction parameters which have been optimized to predict VLE behavior may not provide the best density prediction.
- At several low temperatures, AGA8 did not provide density estimates. The average errors reported here ignored these missing data. Note that AGA8 is not valid for liquid nor for the extended region near the critical point.
- Table 1 indicates that REFPROP and GERG 2004 give equally the best results.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing), G5 (Gas Conditioning and Processing – Special), G6 (Gas Treating and Sulfur Recovery), RF61 (RefineryGas Treating, Sour Water, Sulfur and Tail Gas), PF-81 (CO2 Surface Facilities), and G40 (Process/Facility Fundamentals) courses.
By: Wes Wright and Dr. Mahmood Moshfeghian
References:
- Wichert, E. and Aziz, K., Hydr. Proc., p. 119 (May 1972).
- Lemmon, E.W., Huber, M.L., McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 8.0, National Institute of Standards and Technology, Standard Reference Data Program, Gaithersburg, 2007.
- Kunz, O., Klimeck, R., Wagner, W., and Jaeschke, M. “The GERG-2004 Wide-Range Equation of State for Natural Gases and Other Mixtures,” GERG Technical Monograph 15 (2007)
- Hwang, C-A., Duarte-Garza, H., Eubank, P. T., Holste, J. C. Hall, K. R., Gammon, B. E., March, K. N., “Thermodynamic Properties of CO2 + CH4 Mixtures,” GPA RR-138, Gas Processors Association, Tulsa, OK, June 1995
- Hall, K. R., Eubank, P. T., Holste, J., Marsh, K.N., “Properties of C02-Rich Mixtures Literature Search and Pure C02 Data, Phase I,” GPA RR-68, A Joint Research Report by Gas Processor Association and the Gas Research Institute, Gas Processors Association, Tulsa, OK, June 1985

[…] M., “How good are the detailed methods for sour gas density calculations?,” PetroSkills tip of the month, Oct […]