Process simulation computer programs are excellent tools for designing or evaluating gas processing plants, chemical plants, oil refineries or pipelines. In these simulation programs, most of the thermodynamic properties are calculated by an equation of state (EOS). The cubic equations of state can be regarded as the heart of these programs for generating the required properties. However, none of the equations of state is perfect and often some sort of tuning must be done prior to their applications. Some tuning is already done by researchers and has been embedded in the data base of these simulation programs. In dealing with non-standard or complex systems, the user should check the validity and accuracy of the selected thermodynamic package (i.e. EOS) in the simulation programs prior to attempting to run the desired simulation. Often the users find that tuning is required. This can be done by performing a series of vapor liquid equilibria (VLE) calculations such as dew point, bubble point or flash calculations and comparing the results with the field data or experimental data. If the accuracy is not within acceptable range, then the EOS should be tuned to improve its accuracy. The tuning can be done in several ways but the one most often used is adjusting/regressing the binary interaction parameters between binary pairs in the mixture using the experimental PVT or VLE data.
In this tip of the month (TOTM), we will demonstrate how the binary interaction parameters are tuned in a simulation program to improve the accuracy of a selected EOS. For this purpose, we will demonstrate how the accuracy of the bubble point pressure prediction of a ternary system of carbon dioxide, pentadecane, and hexadecane can be improved. We will use the Peng-Robinson (PR) [1] equation of state in ProMax [2] and the experimental VLE data published in the literature [3]. The same procedure can be used with any EOS in other simulation programs.
The PR EOS
The PR EOS [2] in terms of pressure (P), volume (v) and temperature (T) is defined as:
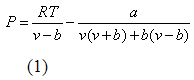
The values of the parameters a and b must be determined in a special way for mixtures. Any equation, or series of equations, used to obtain mixture parameters is called a combination rule or mixing rule. The calculation, regardless of its exact form, is based on the premise that the properties of a mixture are some kind of weighted average summation of the properties of the individual molecules comprising that mixture.
The mixing rules used in cubic equations of state (i.e., Peng-Robinson, Soave-Redlich-Kwong, and van der Waals) are:
Where: a and b = the interaction energy and molecular size parameters for the mixture
ai, bi = a and b parameters for component i in the mixture
xi = composition (mol fraction) for component i in the mixture
kij = binary interaction parameter
n = number of component in the mixture
R = Universal gas constant
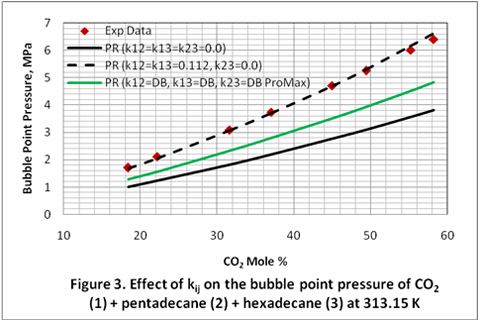
The ai and bi for each component in the mixture are calculated in terms of critical temperature (Tci), pressure (Pci), and acentric factor (?i) as presented in equations 4 and 5.
Once a and b have been determined, the equation of state computations proceed as though a and b were for a pure component. With cubic equations of state the mixing rules sum the properties based on binary pairs.
The binary interaction parameter, kij, has no theoretical basis. It is empirical and is used to overcome deficiencies in the corresponding states theory or the basic model (equation of state). Binary interaction parameters are regressed from experimental data for a specific model and should be applied in that model only. In addition, kij’s can be determined from regression of PVT data or VLE data. This will result in different kij’s for the same binary mixture.
The Effect of kij on Bubble Point Pressure Prediction
To study the effect of the kij, the bubble point pressure for a binary mixture of CO2 (1) and pentadecane (2) at 40 °C for a series of CO2 mole % in the liquid phase were predicted using the PR EOS in ProMax. First, the default value of the binary interaction in the data base (DB) of ProMax in which k12=0.0 was used. The predicted results were compared with the experimental values and the average absolute percent deviation (AAPD) for eight data points calculated to be 41.06%. This AAPD was reduced to 1.64% when the binary interaction parameter of k12=0.112 was used. Figure 1 presents the effect of k12 on the predicted bubble point pressure of CO2 and pentadecane mixture. This figure demonstrates clearly the role of kij in improving the accuracy for bubble point pressure calculations. The improvement is substantial and the accuracy now is as good as the experimental data.
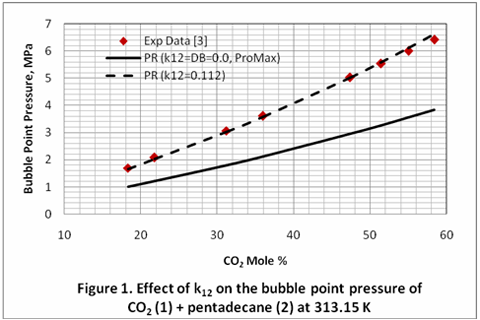
Similar improvement is observed when the binary interaction parameter, k12, was changed from zero, and the default value in data base (k12=DB) of ProMax, to 0.112 for the binary mixture of CO2 (1) and hexadecane (2) at 40 °C. For this case the AAPDs were 40.65%, 3.64% and 1.26% for k12=0.0, k12=DB, and k12=0.112; respectively.
For these two systems the liquid densities were also predicted and compared with the experimental values. For CO2and pentadecane binary system, the calculated AAPD for liquid densities were 6.10% and 6.36% for k12=0.0 and k12=0.112; respectively. Similar AAPD changes were observed for CO2 and hexadecane binary mixture.
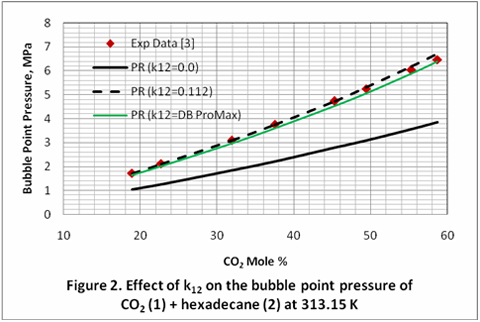
Normally, the binary interaction parameters obtained from regressing binary mixture VLE data work well in multicomponent systems. This is demonstrated by using the same obtained kijs in a ternary mixture. The obtained binary interaction parameters of CO2 + pentadecane and CO2 + hexadecane were used without any further change to predict the bubble point pressure of the ternary mixtures of CO2 (1) + pentadecane (2) + hexadecane (3). Figure 3 indicates these binary interaction parameters obtained from the individual binary mixtures improve the accuracy of EOS considerably. Similar to the case of binary mixtures, when the binary interaction parameters, k12, k13, were changed from zero, and the default value of ProMax data base (kijs=DB), to 0.112 for the ternary mixture of CO2 (1) + pentadecane (2) + hexadecane (3) at 40 °C, the AAPDs were reduced from 40.99% and 25.16% to 1.75%, respectively.
Discussion and Conclusions
It was shown that the binary interaction parameters of an EOS can be adjusted/tuned/regressed to improve the accuracy of VLE calculations considerably. It was also shown that when the regressed binary interaction parameters based on the binary experimental VLE data used without further changes in a multicomponent system considerable improvement in accuracy may be obtained.
It is a sound practice to check the accuracy of a selected thermodynamic package prior to running any simulation. However, experimental or field data are required to fulfill this task.
To learn more about similar cases and how to run process simulations, we suggest attending our G40(Process/Facility Fundamentals), G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special) courses.
By: Dr. Mahmood Moshfeghian
References:
- Peng, D.Y. and Robinson, D.B., “A New Two-Constant Equation of State,” Ind. Eng. Chem., Fundam., Vol. 15, No. 1, P. 59, 1976.
- ProMax, V. 3.0, Bryan, Tex.: Bryan Research & Engineering Inc, 2009.
- Tanaka, H., Yamaki, Y. and Kato, M., “Solubility of Carbon Dioxide in Pentadecane, Hexadecane, and Pentadecane + Hexadecane,” J. Chem. Eng. Data,38, 386-388,1993.

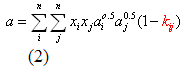
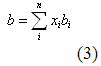
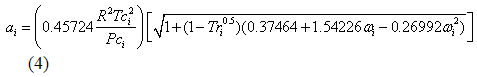
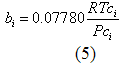
Very good information. Lucky me I found your website by accident (stumbleupon). I’ve saved it for later!
A very informal and articulate disquisition by Dr Mahmood. I’ve been very confused about EOS & it’s relationship with BIP. Now it’s crystal clear. Bundle of thanks again Dr. Mahmood. Best regards, Muhammad Shehreyar.
I like this site so much, saved to fav. “To hold a pen is to be at war.” by Francois Marie Arouet Voltaire.
I visited a lot of website but I believe this one has got something extra in it.