Hydrogen blistering is a type of hydrogen-induced failure produced when hydrogen atoms enter low-strength steels that have macroscopic defects, such as laminations. The defects in the steel (void spaces) provide places for hydrogen atoms to combine, forming gaseous molecular hydrogen (H2) that can build enough pressure to produce blistering. Hydrogen blistering is a problem mainly in sour environments. It does not cause a brittle failure, but it can produce rupture or leakage [1]. Description and mechanisms of hydrogen blistering can be found in literature [2]. Hydrogen sulfide concentration, temperature and thickness of material affect hydrogen blistering.
In this TOTM we will consider the quantitative effect of temperature and hydrogen sulfide mole fraction causing hydrogen damage in the fractionation columns of an operating natural gas liquid (NGL) Plant [3]. The fractionation unit was designed to process a broad-cut of NGL, which is an off-product from crude oil production units and produces essentially propane, butane, and natural gasoline. The feed to the process is introduced into the fractionation unit where propane, butane and gasoline are separated by three distillation columns. In the first column, which is a deethanizer, ethane and lighter compounds are separated from the feed stream. In the second column, a depropanizer, propane is fractionated and sent to the amine treater for further processing to meet market specifications. The bottoms of the depropanizer are fed to the third column, a debutanizer, in which butane is distilled over and sent to a Merox unit for further treating. The bottoms of the debutanizer column, essentially gasoline, are also sent to Merox for treating. More information on NGL production technologies can be found in reference [4].
During the overhaul of this NGL Plant, the inspection team found that the deethanizer-reflux-accumulator had been damaged due to severe hydrogen blistering in the shell and bottom plate, and the vessel was rejected. Four years later, during an inspection of the Plant, the deethanizer rectifying section and depropanizer rectifying section were found to have been severely damaged by hydrogen blistering.
In order to study the effects of hydrogen sulfide and local temperature quantitatively and more closely, the three distillation columns in a fractionation unit were simulated. In this simulation, which could assist one to thoroughly understand the causes of hydrogen attack, the values of temperature and hydrogen sulfide mole fraction along each column were determined by performing tray-by-tray calculations.
Case Study:
The operating NGL Plant consists of fractionation, treating, drying, refrigeration, utility, storage and loading facilities to process approximately 57,700 barrels (9172 m3) of broad-cut NGL per day. The charge to this plant is essentially Natural Gasoline Liquid which is condensed out of oil-field gas and off-product from several crude oil production units. The broad-cut is processed to produce propane, butane and light gasoline (Pentane Plus Product). The feed stream also contains some impurities such as hydrogen sulfide, carbon dioxide and mercaptan, which are removed by treating the products after fractionation.
Since hydrogen blistering occurred only in the fractionation unit, a brief description of this unit is given in the following section [3].
A schematic flow diagram for this unit is given in Figure 1. The 40-tray deethanizer tower receives raw feed from NGL recovery plants, fractionates out ethane and lighter products and delivers essentially ethane-free NGL to the depropanizer column. The feed to the deethanizer is introduced between the 27th and 28th trays at 135°F and 362 Psig (57.2 °C and 2497 kPag). Bottoms product from the deethanizer is charged to tray 22 of a 45-tray depropanizer column. The distillate product, which is essentially propane, is sent to the amine treater unit for further processing. The depropanizer bottoms are charged to tray 20 of the 40-tray debutanizer column. The debutanizer distillate product, which comprises the net butane product, is sent to the Merox plant for treating. The debutanizer bottom (pentane and heavier products essentially free of butane) is also sent to the Merox plant for further processing.
Figure 1. Flow diagram of fractionation unit
The following information was specified for simulation of the fractionation unit:
(i) Flow diagram as shown in Figure 1
(ii) Feed stream condition and composition as shown in Table 1
(iii) Column specification as presented in Table 2
Other specifications such as a desired percentage recovery of a component in any product stream, could have been used instead of the reflux ratio or bottoms product ratio. In the course of simulation, tray by tray calculations were performed to calculate temperature, pressure, vapor and liquid compositions, and vapor and liquid traffics for each tray in each column. In addition, distillate and bottoms rates, temperature, pressure, composition, reboiler and condenser duties were also calculated, as were height and diameter of the columns.
To perform the simulation, Vapor Liquid Equilibrium K-values, liquid and vapor enthalpies were computed by the Peng-Robinson equation of state [5]. In the tray-by-tray calculation it was assumed that the trays performed ideally (100 % efficiency). The simulation was carried out by UniSim simulation software [6]
Table 1. Feed stream composition and specification
| Component | Mole % |
| CO2 | 1.167 |
| H2S | 0.325 |
| Methane | 5.625 |
| Ethane | 15.724 |
| Propane | 28.190 |
| i-Butane | 6.724 |
| n-Butane | 17.812 |
| i-Pentane | 5.812 |
| n-Pentane | 6.846 |
| n-Hexane | 5.998 |
| C7+ | 5.777 |
| T, °F (°C) | 135.0 (57.2) |
| P, Psig (kPag) | 362.0 (2497) |
| Rate, lbmole/hr (kmole/h) | 8619 (3909) |
Table 2. Fractionation towers specifications
| Column | Pressure, Psig (kPag) | No of Trays | Feed Tray from Bottom | Reflux Ratio, L/F | Bottoms Ratio, B/F | Condenser Type | ||
| Feed | Condenser | Reboiler | ||||||
| Deethanizer | 362 (2497) | 347(2393) | 360 (2483) | 40 | 27 | 0.4438 | 0.7749 | Partial |
| Depropnizer | 300(2069) | 290(2000) | 300 (2069) | 45 | 22 | 1.0709 | 0.6415 | Total |
| Debutanizer | 95 (655) | 85(586) | 95 (655) | 40 | 20 | 1.0082 | 0.4889 | Total |
Results and Discussion:
Performing a simulation, a great deal of information is produced. However, only information of interest in regard to hydrogen blistering is presented here. To test the validity of the simulation results, composition and condition of the key process streams are compared with those supplied by the designer of the plant [7] and presented in Table 3. In most cases the results compare favorably. In addition, condenser and reboiler duties for each column are compared with the original design values in Table 4. This comparison of the two sets of results shows a maximum deviation of –13.3% for the depropanizer reboiler. With the exception of the deethanizer boiler, all of the design heat exchange duties are higher than those obtained in this simulation, which is, of course, a normal safeguard in plant design.
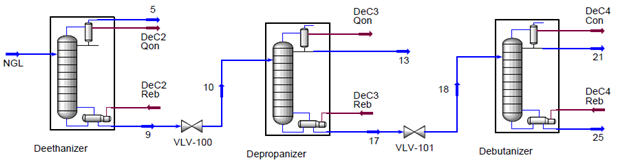
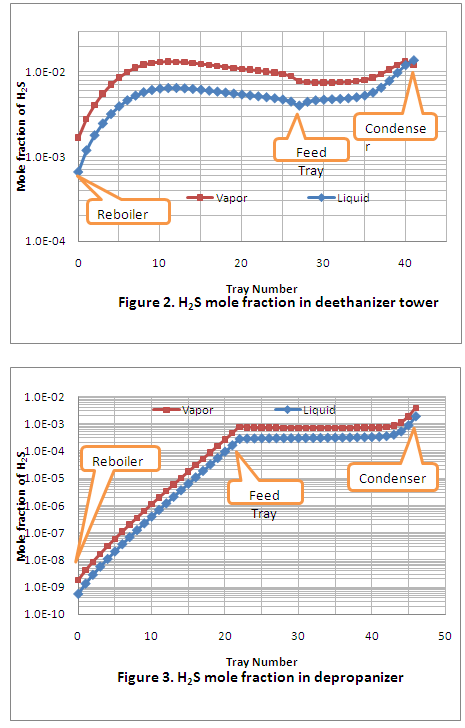
Table 3 shows hydrogen sulfide is fractionated in the first two columns and does not reach the debutanizer column. Since hydrogen blistering occurred in the first two columns, only these results were examined closely. To study the variation of hydrogen sulfide composition (in both liquid and vapor phases) along each column, its composition is plotted as a function of tray number. This is shown in Figure 2 for the deethanizer and Figure 3 for the depropanizer.
Table 3. Comparison of simulation results and design data for process streams leaving fractionation towers
| Component | Stream 5 | Stream 13 | Stream 21 | Stream 25 | ||||
| Simulation | Design | Simulation | Design | Simulation | Design | Simulation | Design | |
| CO2 | 5.183 | 5.184 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| H2S | 1.213 | 1.149 | 0.185 | 0.208 | 0.000 | 0.000 | 0.000 | 0.000 |
| Methane | 24.983 | 24.991 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ethane | 65.138 | 66.171 | 3.812 | 2.994 | 0.000 | 0.000 | 0.000 | 0.000 |
| Propane | 3.483 | 2.505 | 92.460 | 95.808 | 6.779 | 3.987 | 0.000 | 0.000 |
| i-Butane | 0.000 | 0.000 | 3.244 | 0.890 | 22.918 | 25.484 | 0.002 | 0.011 |
| n-Butane | 0.000 | 0.000 | 0.298 | 0.100 | 69.276 | 69.542 | 0.529 | 0.477 |
| i-Pentane | 0.000 | 0.000 | 0.000 | 0.000 | 0.990 | 0.923 | 22.880 | 22.951 |
| n-Pentane | 0.000 | 0.000 | 0.000 | 0.000 | 0.037 | 0.064 | 28.133 | 28.105 |
| n-Hexane | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 24.682 | 24.682 |
| C7+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 23.773 | 23.774 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| T, F | 16.4 | 20.0 | 134.5 | 141.0 | 131.2 | 144.0 | 264.6 | 273.0 |
| T, C | -8.7 | -6.7 | 56.9 | 60.6 | 55.1 | 62.2 | 129.2 | 133.9 |
| P, psig | 347 | 290 | 85 | 95 | ||||
| P, kPa(g) | 2393 | 2000 | 586 | 655 | ||||
| Rate, lbmole/hr | 1940.4 | 1939.8 | 2394.1 | 2394.1 | 2189.6 | 2189.6 | 2094.5 | 2094.5 |
| Rate, kmole/h | 880.2 | 879.9 | 1086.0 | 1085.9 | 993.2 | 993.2 | 950.1 | 950.0 |
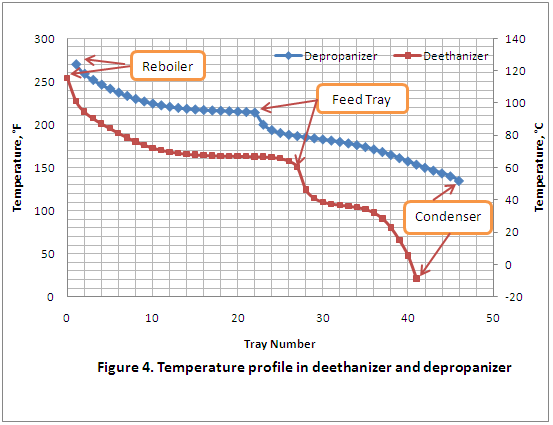
Similarly, in Figure 4, the temperature variation along these two columns is plotted as a function of tray number, and it can be seen that the temperature profiles decrease smoothly from bottom to top except in the feed zone, which is to be expected in a distillation column with no side draw or inter-stage reboiler/cooler.
Figure 2 indicates that the maximum mole fraction of hydrogen sulfide occurred on tray 11 in the stripping section of the deethanizer while hydrogen blistering occurred in the rectifying section. Therefore, other factors such as temperature must be influencing the hydrogen damage. In the stripping section where no hydrogen blistering occurred, the temperature was higher than in the rectifying section where hydrogen blistering was detected. Another region where hydrogen blistering was found is the top part of the depropanizer rectifying section. In this section of the column, the hydrogen sulfide mole fraction is almost the same as in the stripping section of the deethanizer; however the temperatures for these two sections are not the same. The temperature range for the deethanizer stripping section is 142° to 240°F (61.1 to 115.6°C), and for the troubled region of the depropanizer, it is 142° to 134° F (61.1 to 56.6°C), trays 44, 45 and the condenser. Again, it can be seen how temperature influences the hydrogen blistering damage process. In this case, the hydrogen blistering was occurring at lower temperatures. Simulation results also indicate that carbon dioxide does not reach the depropanizer and debutanizer.
Conclusions:
Based on the simulation results and preceding discussion, the following conclusions can be made:
1- Hydrogen blistering can occur where hydrogen sulfide is present. In the case studied a mole fraction of as low as 0.002 for hydrogen sulfide caused hydrogen damage.
2- With the presence of hydrogen sulfide, temperature is the important factor promoting hydrogen blistering. In the case studied a temperature of less than 142°F (61.1°C) caused hydrogen damage. Higher temperature drives hydrogen out of the wall to atmosphere.
There are probably other factors governing hydrogen damage such as microstructure of materials, thickness of material, presence of CO2, etc. Even though the simulation was performed based on a dry feed, the actual feed to the plant contained some water.
The above results are consistent and the same as those reported by the author in 1985 [3]. In the original work, the simulation was carried out by a computer package named Process Analysis System (PAS) developed by Erbar and Maddox [8]. At that time, the computations were made on an IBM 370 main frame at Shiraz University Computing Center. The Soave-Redlich-Kwong [9] equation of state was used in the original work.
A heat exchanger failure at the Tesoro Anacortes refinery was determined to have experienced a form of hydrogen blistering. That failure led to the deaths of seven workers and the refinery was shut down for over six months to repair the damage. It was determined that the root cause of the failure was hydrogen blistering in the steel of the heat exchanger which resulted in rupture. These types of hydrogen attacks can be discovered during scheduled inspections. If there is a concern that conditions are conducive to hydrogen blistering, one can use a hydrogen patch probe to measure hydrogen activity within metals. If hydrogen activity is found in the metal, then additional testing can be completed to determine if any internal cracks have developed.
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses; G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing-Special).
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- http://www.glossary.oilfield.slb.com/Display.cfm?Term=hydrogen%20blistering
- Mostert, R., and Sharp, W.R., “Low Temperature Hydrogen Damage Assessment in the Gas and Refining Industries,” 3rd Middle East Nondestructive Testing Conference & Exhibition – Bahrain, Manama, 27-30 Nov 2005.
- Moshfeghian, M., “Hydrogen damage (Blistering) case study: Mahshahr NGL Plant”, Iranian J. of Science & Technology, Vol 11, No.1, 1985.
- Campbell, J. M., “Gas Conditioning and Processing”, Vol. 2, The Equipment Module, 8th Ed., Second Printing, J. M. Campbell and Company, Norman, Oklahoma, 2002
- Peng, D. Y. and Robinson, D. B., I. and E. C. Fund, Vol. 15, p. 59, 1976.
- UniSim Design R390.1, Honeywell International, Inc., Calgary, Canada, 2010.
- Parsons, R. M., NGL Fractionation Facilities, Operation Manual Bandar Mahshahr, The Ralph M. Parsons Company U. K. Ltd.
- Erbar, J. H., and Maddox, R. N., Process Analysis System, Documentation, Oklahoma State University, Stillwater OK., 1978.
- Soave, G., Chem. Eng. Sci. Vol. 27, No. 6, p. 1197, 1972.

This is my first time pay a visit at here and i am in fact impressed to
read all at single place.
Magnificent beat ! I would like to apprentice while you amend
your web site, how could i subscribe for a blog web site? The account aided me a
acceptable deal. I had been a little bit acquainted of
this your broadcast offered bright clear concept
A great read. Just some small things to make the article mistake free.
1. I think the de-ethanizer column is Full Reflux and not Partial Reflux as specified in table 2.
2. There is a reference of Table 4 in the article but I don’t see any table 4 here.
Thank you.