Glycol dehydration is the most common dehydration process used to meet pipeline sales specifications and field requirements (gas lift, fuel, etc.). Triethylene glycol (TEG) is the most common glycol used in absorption systems. Chapter 18, Gas Conditioning and Processing [1] presents the process flow diagram and basics of glycol units. A key parameter in sizing the TEG dehydration unit is the water dew point temperature of dry gas leaving the contactor tower. Once the dry gas water dew point temperature and contactor pressure are specified, water content charts similar to Figure 1 in reference [2] can be used to estimate the water content of lean sweet dry gas. The required lean TEG concentration is thermodynamically related to the dry gas water content which influences the operating (OPEX) and capital (CAPEX) costs. The lower dry gas water content requires a higher lean TEG concentration. This parameter sets the lean TEG concentration entering the top of contactor and the required number of trays (or height of packing) in the contactor tower.
The rich TEG solution is normally regenerated at low pressure and high temperature. Maximum concentrations achievable in an atmospheric regenerator operating at a decomposition temperature of 404 °F (206°C) is 98.7 weight %. The corresponding dry gas water dew point temperature for this lean TEG weight % and contactor temperature of 100°F (38°C) is 18°F (-8°C).
If the lean glycol concentration required at the absorber to meet the dew point specification is higher than the above maximum concentrations, then some method of further increasing the glycol concentration at the regenerator must be incorporated in the unit. Virtually all of these methods involve lowering the partial pressure of the glycol solution either by pulling a vacuum on the regenerator or by introducing stripping gas into the regenerator.
For water saturated gases, the water dew point temperature is either above or at the hydrate formation temperature. However, if the gas is water under-saturated, the hydrate formation temperature will be higher than water dew point. This means at a given specified water dew point temperature, there are two water content values; the lower value will be at the hydrate formation temperature and the higher value will be at the water dew point temperature. Therefore, the designer has to choose one of these two values. Which value should be chosen? The answer to this question is “It depends”! The lower value of water content means higher lean TEG concentration and consequently higher CAPEX and OPEX.
In this TOTM we will attempt to answer the question by studying a case in which the specified water dew point temperature is below the hydrate formation temperature. For this purpose, we will discuss the water content of natural gas in equilibrium with hydrate and when the condensed water phase is liquid.
The water content chart of Figure 6.1 in reference [2] is based on the assumption that the condensed water phase is a liquid. However, at temperatures below the hydrate temperature of the gas, the “condensed” phase will be a solid (hydrate). The water content of a gas in equilibrium with a hydrate will be lower than equilibrium with a metastable liquid.
Hydrate formation is a time dependent process. The rate at which hydrate crystals form depends upon several factors including gas composition, presence of crystal nucleation sites in the liquid phase, degree of agitation, etc. During this transient “hydrate formation period” the liquid water present is termed “metastable liquid.” Metastable water is liquid water which, at equilibrium, will exist as a hydrate.
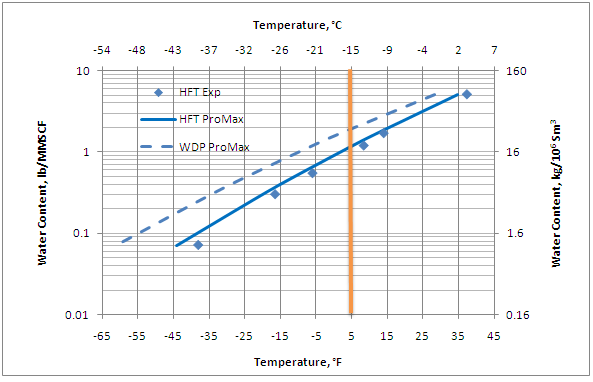
Reference [3] presents experimental data showing equilibrium water contents of gases above hydrates. Data from Reference [3] are presented in Figure 6.5 of reference [2] and plotted here as rotated square in Figure 1 at 1000 Psia (6,897 kPa). For comparative purposes, the “metastable” water content of the gas (dashed line) as well as the hydrate formation temperature (solid line) calculated by ProMax [4] using the Peng-Robinson [5] equation of state are also shown. The water content of gases in the hydrate region is a strong function of composition. Figure 1 should not be applied to other gas compositions.
Figure 1. Water content of 94.69 mole % methane and 5.31 mole % propane – gas in equilibrium with hydrate at 1000 Psia (6,897 kPa)
Case Study:
To demonstrate, the effect of water content of a dried gas in equilibrium with hydrate on the required lean TEG concentration, let’s consider the gas mixture presented in Figure 1. This gas enters a contactor tower at 1000 Psia (6,897 kPa) and 100 °F (37.8°C) with a rate of 144 MMSCFD (4.077 106 Sm3/d). At this condition, the water content of the wet gas is 57.6 lb/MMSCF (922.4 kg/106 Sm3). It is desired to dehydrate the gas to a water dew point temperature of 5°F (-15°C) using a TEG dehydration unit.
Results and Discussion:
According to Figure 1, at a temperature of 5°F (-15°C) the water content is 1.2 lb/MMSCF (19.2 kg/106 Sm3) and 1.97 lb/MMSCF (31.5 kg/106 Sm3) in equilibrium with metastable water and hydrate phase, respectively. ProMax was used to simulate this TEG dehydration unit for the case of three theoretical trays in the contactor tower. The simulation results for these two water content cases are shown in Table 1. This table clearly indicates that the required lean TEG concentrations are not the same and consequently will impact the regeneration requirements of the rich TEG solution. The difference between the lean TEG concentrations will be even more at a lower dry gas water dew point specification.
The simulation results clearly indicate that the choice of water content for a specified dry gas water dew point as the basis for design affects the required lean TEG concentration and consequently the rich TEG solution regeneration requirements.
Table 1. Comparison of simulation results for two different water content specifications
| Simulation Results Using ProMax | Based on Water Dew Point Temperature of 5 °F (-15°C) | Based on Hydrate Formation Temperature of 5 °F (-15°C) |
| Water Dew Point Temperature , °F (°C) | 5.0 (-15.0) | -6.2 (-21.2) |
| Hydrate Formation Temperature, °F (°C) | 14.7 (-9.6) | 5.0 (-15.0) |
| Water Content, lb/MMSCF (kg/106 Sm3) | 1.97 (31.5) | 1.20 (19.2) |
| Gallon/lb of Water Removed (liter/kg of Water Removed) | 3.95 (32.9) | 3.90 (32.4) |
| Lean TEG Weight % | 99.45 | 99.72 |
Conclusions:
When designing dehydration systems, particularly TEG systems to meet extremely low water dew point specifications, it is necessary to determine the water content of the dried gas in equilibrium with a hydrate using a correlation like that presented in Figure 1. If a metastable correlation is used, one will overestimate the saturated water content of the gas at the dew point specification. This, in turn, may result in a dehydration design which is unable to meet the required water removal. Where experimental data is unavailable, utilization of an EOS-based correlation which has been tuned to empirical data can provide an estimate of water content in equilibrium with hydrates.
To meet pipeline sales specifications, it is normally acceptable to use the water content in equilibrium with the metastable phase (the dashed line in Figure 1) because the difference in the water contents is not that high. However, for extremely low water dew point specifications where there is a cryogenic process downstream, it is recommended to use the water content in equilibrium with hydrate (the solid line in Figure 1).
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses: G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing-Special).
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- Campbell, J. M., “Gas Conditioning and Processing”, Vol. 2, The Equipment Module, 8th Ed., Second Printing, J. M. Campbell and Company, Norman, Oklahoma, 2002
- Campbell, J. M., “Gas Conditioning and Processing”, Vol. 1, The Basic Principles, 8th Ed., Second Printing, J. M. Campbell and Company, Norman, Oklahoma, 2002
- Song, K.Y. and Kobayashi, R, “Measurement & Interpretation of the Water Content of a Methane-5.31 Mol% Propane Mixture in the Gaseous State in Equilibrium With Hydrate,” Research Report RR-50, Gas Processors Association, Tula, Oklahoma, 1982
- ProMax 3.1, Bryan Research and Engineering, Inc, Bryan, Texas, 2010.
- Peng, D. Y. and Robinson, D. B., I. and E. C. Fund, Vol. 15, p. 59, 1976.

please correct me if i’m wrong, you mentioned that “According to Figure 1, at a temperature of 5°F (-15°C) the water content is 1.2 lb/MMSCF (19.2 kg/106 Sm3) and 1.97 lb/MMSCF (31.5 kg/106 Sm3) in equilibrium with metastable water and hydrate phase, respectively.”.it seems that 1.97 lb/MMSCF should be for metastable and 1.2 lb/MMSCF for hydrate, in contrast to aforementioned.