In a past Tip Of The Month (TOTM), we have shown that one of the first issues to be resolved by a facilities engineer working in a gas plant or gas production facility is where the process is operating with respect to the phase diagram. A general knowledge, if not a detailed knowledge, will allow the design engineer and the facilities operator to make intelligent decisions that have significant impact on the profitability of a gas production facility.
The best way to prevent hydrate formation (and corrosion) is to keep the pipelines, tubing and equipment dry of liquid water. In this TOTM we will demonstrate how the water dew point and hydrate formation curves are shifted along a conventional phase envelope as natural gas is dehydrated.
Case Study:
In order to demonstrate the phase behavior of natural gases containing water and the impact of water content on the water dew point and hydrate formation temperatures, let’s consider the natural gas shown in Table 1. To generate the diagrams in this TOTM, we used ProMax [1] based on the Peng-Robinson equation of state (PR EOS) [2].
Table 1. Dry gas composition
| Component | Mole % |
| C1 | 80.0 |
| C2 | 10.0 |
| C3 | 4.0 |
| iC4 | 3.0 |
| nC4 | 3.0 |
| Sum | 100.0 |
Results and Discussion:
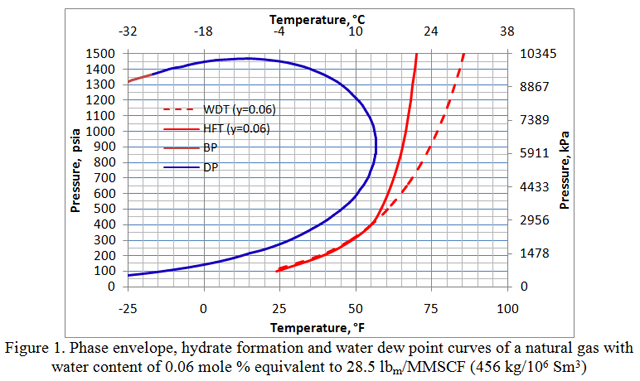
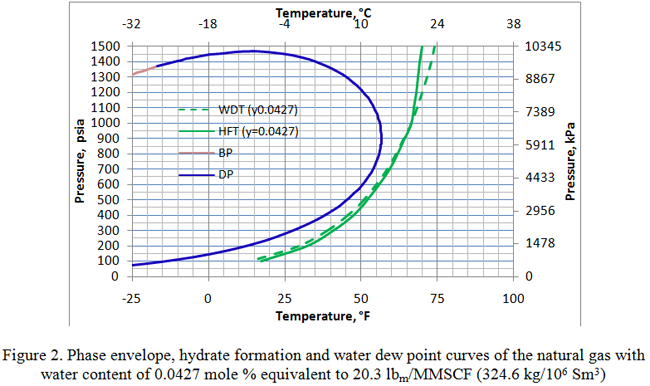
Figure 1 presents the phase envelope, hydrate formation and water dew point curves of this gas with a water content of 0.06 mole percent, equivalent to 28.5 lbm/MMSCF (456 kg/106 Sm3). Notice that up to a pressure of about 414 psia (2854 kPa), the water dew point curve is slightly to the left of the hydrate formation curve. This indicates that the gas is under-saturated with water at pressures below this point. This also means that it is thermodynamically unstable and will not form a free aqueous phase. All the water is converted to hydrate and this state is referred to as “meta-stable” equilibrium. For more detail on this meta-stable state, see December 2010 TOTM. Similar behavior is demonstrated in Figure 2 for which the water content was reduced to 0.0427 mole percent, equivalent to 20.3 lbm/MMSCF (324.6 kg/106 Sm3). In this case the water dew point and hydrate formation curves intersect at a higher pressure of 1000 psia (6895 kPa). Below this pressure, the gas is under-saturated and has a meta-stable equilibrium state. Therefore, the water dew point curve is to the left of the hydrate formation curve, but above the intersection pressure it moves to the right of the hydrate formation curve where the water content is above saturation.
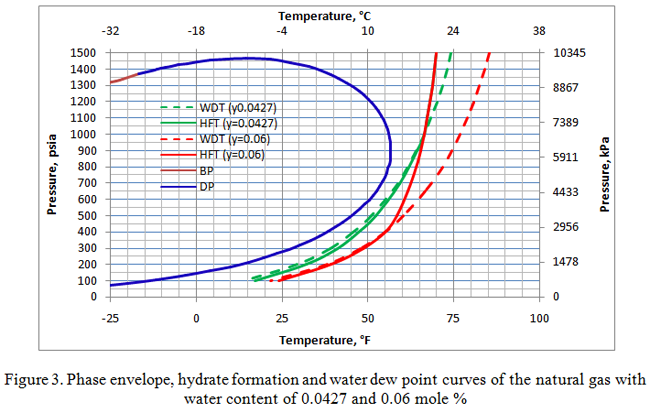
Figure 3 presents the superimposition of Figures 1 and 2 having water dew point and hydrate formation curves for two different water contents (0.06 and 0.0427 mole%). Notice the hydrate formation curves for both cases coincide with each other for pressures of 1000 psia (6895 kPa) and higher.
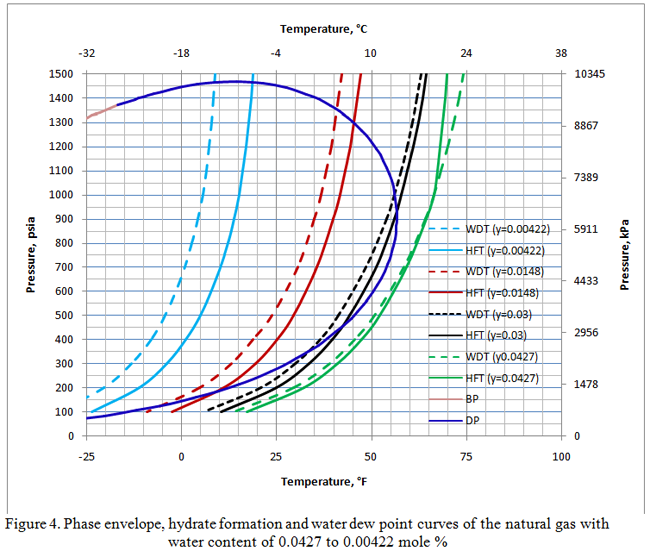
Figure 4 presents the phase envelope along with the water dew point and hydrate formation curves for the same gas as the water content was reduced to 0.0427, 0.03, 0.0148, and 0.00422 mole % corresponding to 20.3, 14.2, 7, 2 lbm/MMSCF (324.6, 228, 112, 32 kg/106 Sm3), respectively. Notice for all the cases where the gas is under-saturated with water, the water dew point curves are located to the left of the corresponding hydrate formation curves. Under these conditions the equilibrium state is thermodynamically unstable (meta-stable) and will not form a free aqueous phase. However, if the water content is above saturation point, then the water dew point will position to the right of the corresponding hydrate formation curve and free water will form under stable condition.
Conclusions:
We have demonstrated the impact of the water content on the phase behavior of a natural gas. The emphasis was placed on the interaction of the water dew point and hydrate formation curves. It was shown that the relative location of the water dew point and hydrate curves with respect to each other is a strong function of the water content. It was also shown for the cases where water content is above saturation point, the water dew point curve locates to the right of the hydrate curve. Under this condition free water forms and then hydrates may form if conditions are right. This is what is normally expected and shown in text books. However, if the water content is under-saturated, the water dew point curve will be located to the left of the hydrate formation curve and the equilibrium state is thermodynamically unstable (meta-stable) and will not form a free aqueous phase.
As discussed in last month’s TOTM, facility engineers have to determine how this behavior affects their operations. These phase envelopes suggest that, at low water concentrations, hydrates may form even though free water is not present. Indeed, this phenomenon has been observed. At cryogenic conditions, when the water is removed by molecular sieves, the amount of metastable water is so small it should not cause operational issues.
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses; G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing-Special).
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- ProMax 3.2, Bryan Research and Engineering, Inc, Bryan, Texas, 2010.
- Peng, D. Y. and Robinson, D. B., I. and E. C. Fund, Vol. 15, p. 59, 1976.

i have problem to make code to estimate dew point pressure ,temperature and bubble point temperature using cubic equation of state
but in bubble point pressure the code run and return good resuls
pleas anyone help me
Thank you a loot for sharing this with all of us you really realize what
you’re speaking about! Bookmarked. Kindly also consult with my site =).
We maay have a hyperlink alternate contract among us
Hi, i think that i saw you visited my weblog so i got
here to go back the choose?.I am trying to in finding issues
to enhance my site!I assume its good enough to use a few of your ideas!!
hi!,I love your writing very soo much! share we commmunicate more approximately your post
on AOL? I require a specialist on thks space to unravel my problem.
Maybe that’s you! Having a look ahead to see you.
There is no more.
Hi I am so glad I found your website, I really found you by error, while I was looking on Askjeeve for something else, Regardless I am here now and would just like to say thanks a lot for a marvelous post and a all round thrilling blog (I also love the theme/design), I don’t have time to browse it all at the minute but I have book-marked it and also included your RSS feeds, so when I have time I will be back to read a lot more, Please do keep up the superb work.
Thanks for sharing your valuable knowledge.
I was wondering if you could answer me the question that I have, which is
“When the gas without free water is transforted through the gas export pipeline, could the water in the gas phase drops out and remains in the pipeline?”
Regards,
Thanks for sharing. I have another perspective that perhaps you can help guide. As a seller, would I prefer to sell my gas at 2lb/mmscf or 6lb/mmscf? Would gas at higher water content weight more? Is it acceptable to operate the plant at 6lb/mmscf from financial point of view?
Please send me this information to my email account , thanks,
Steve Moseley
Maintenance coordinator
Pecos bend gas plant
Pecos Texas 77982
If pipeline natural gas has P1 = 1050 psi , T1 = 80 degree F and water content = 6.8 lbs/mmscf , How much water content when dropped it to P2 = 200 psi and T2 = 36 degree F
Thanks and Regards
Worawith S
I want to receive posts by email.
It seems Thermodynamics models are unable to accurately predict the formation dynamic of hydrates. Additionally, kinetics of nano- to micro-droplet growth might control the early stages of this complex process.