In the January and February 2012 tips of the month (TOTM) we discussed the isothermal and non-isothermal transportation of pure carbon dioxide (CO2) in the dense phase region. We illustrated how thermophysical properties changed in the dense phase and studied their impacts on pressure drop calculations. The pressure drop calculation results utilizing the liquid phase and vapor phase equations were exactly the same. We showed that the effect of the overall heat transfer coefficient on the pipeline temperature is significant. In this TOTM, we will study the same case study in the presence of nitrogen impurities under non-isothermal conditions. The Joule-Thompson expansion effect and the heat transfer between pipeline and surroundings have been considered. Specifically, we will report the effect of nitrogen impurities on the pressure and temperature profiles. The Peng-Robinson equation of state (PR EOS) was utilized in this study.
For a pure compound above critical pressure and critical temperature, the system is often referred to as a “dense fluid” or “super critical fluid” to distinguish it from normal vapor and liquid (see Figure 1 for carbon dioxide in December 2009 TOTM [1]).
Calculation Procedure:
The same step-by-step calculation procedure described in the February 2012 TOTM [2] was used to determine the pressure and temperature profiles in a pipeline considering the Joule-Thompson expansion effect and heat transfer between the pipeline and surroundings.
In the following section we will illustrate the pressure drop calculations for transporting CO2 in dense phase using the gas phase pressure drop equations. For details of pressure drop equations in the gas and liquid phases refer to the January 2012 TOTM [3].
Case Study:
For the purpose of illustration, we considered a case study [also described in reference 2] for transporting 160 MMSCFD (4.519×106 Sm3/d) CO2 using a 100 miles (160.9 km) long pipeline with an inside diameter of 15.551 in (395 mm). The inlet conditions were 2030 psia (14 MPa) and 104˚F (40˚C). The following assumptions were made:
- CO2, with nitrogen impurities of 0, 1, 5, 10, and 15 mole %.
- Horizontal pipeline, no elevation change.
- Inside surface relative roughness’s (roughness factor), ε/D, of 0.00013.
- The ambient/surrounding temperature,Ts, is 55 ˚F and (12.8 ˚C)
- Overall heat transfer coefficients of 0.5 Btu/hr-ft2-˚F (2839 W/m2-˚C).
Properties: The dense phase behavior and properties were calculated using the Peng-Robinson equation of state (PR EOS) [4] in ProMax [5] software. ProMax was also used to determine pressure and temperature profiles along the pipeline.
Results and Discussions:
Figures 1 through 4 present the phase envelope, dry ice (CO2 freeze out) curve, and pipeline pressure and temperature profile for 1, 5, 10, and 15 mole % N2 impurities, respectively, the relative roughness (ε/D) of 0.00013, and the overall heat transfer coefficient (U) of 0.5 Btu/hr-˚F-ft2 (2.839 W/m2-˚C).
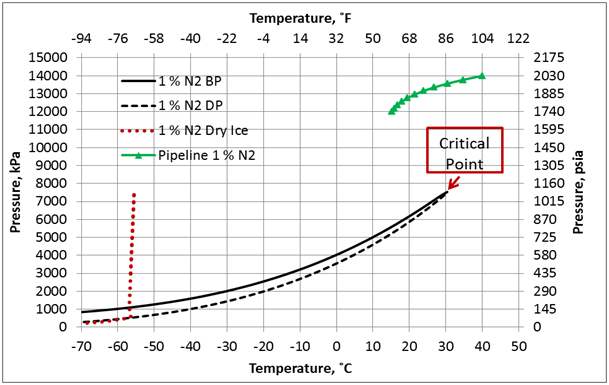
Figure 1. Phase envelop and dense phase pipeline pressure-temperature profile for 99 mole % CO2 + 1 mole % N2, ε/D=0.00013, and U=0.5 Btu/hr-˚F-ft2 (2.839 W/m2-˚C).
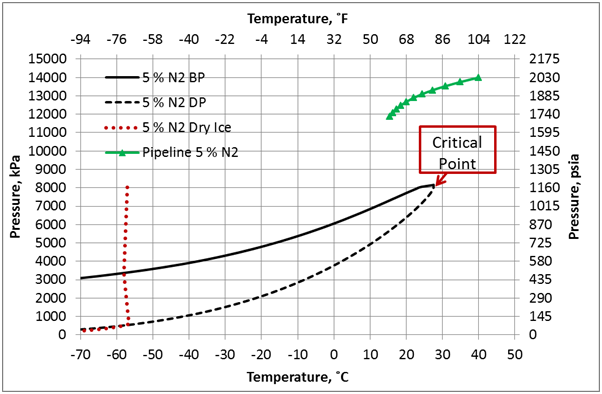
Figure 2. Phase envelop and dense phase pipeline pressure-temperature profile for 95 mole % CO2 + 5 mole % N2, ε/D=0.00013, and U=0.5 Btu/hr-˚F-ft2 (2.839 W/m2-˚C).
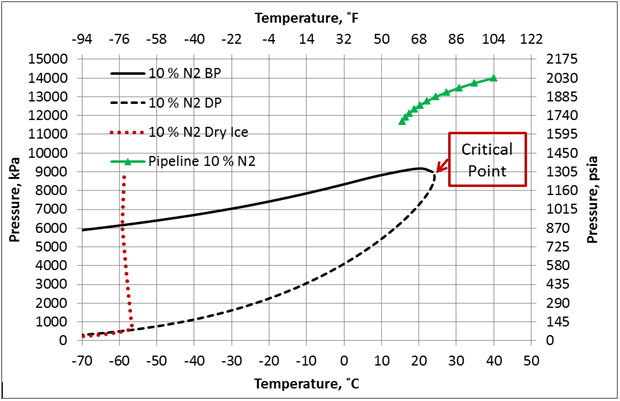
Figure 3. Phase envelop and dense phase pipeline pressure-temperature profile for 90 mole % CO2 + 10 mole % N2, ε/D=0.00013, and U=0.5 Btu/hr-˚F-ft2 (2.839 W/m2-˚C).
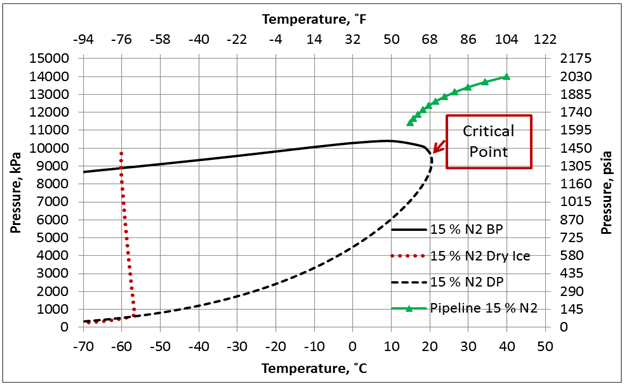
Figure 4. Phase envelop and dense phase pipeline pressure-temperature profile for 85 mole % CO2 + 15 mole % N2, ε/D=0.00013, and U=0.5 Btu/hr-˚F-ft2 (2.839 W/m2-˚C).
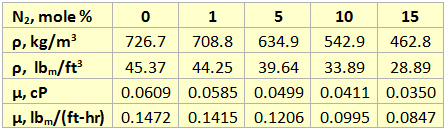
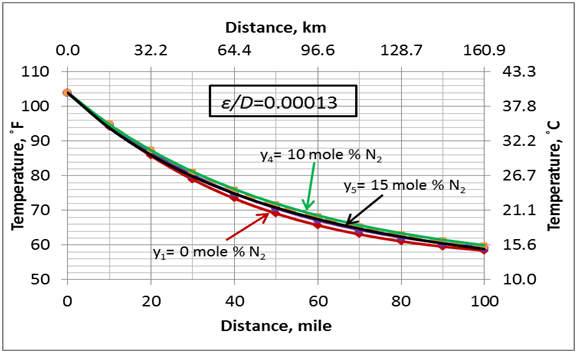
The effect of N2 impurities on the line temperature profile is shown in Figure 5. This figure indicates that N2 impurities have negligible effect on the pipeline temperature profile.
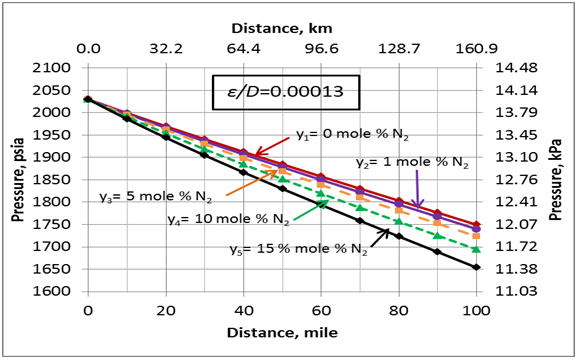
Figure 6 presents the effect of N2 impurities on the pipeline pressure profile. This figure indicates that as the N2 impurities increases the pressure drop increases. This can be explained by the fact as the N2 impurities increase, the mixture density decreases, consequently the velocity increases. Note the pressure drop is proportional to square of velocity and inverse of density. While viscosity decreases with increase in N2 impurities, its effect is not as large as the density effect. Table 1 presents variation of the mixture density and viscosity as a function of N2 mole %.
Table 1. Effect of N2 impurities on density (ρ) and viscosity (µ) of mixture at the inlet condition of 2030 psia (14 MPa) and 104˚F (40˚C)
Conclusions:
Analyzing Table 1 and Figures 1 through 6, the following conclusions can be made:
- For the range 0 to 15 mole % N2, the effect of the N2 impurities on the pipeline temperature profile is negligible.
- As the N2 impurities increase, the pipeline pressure drop increases due to the change in thermophysical properties of mixture.
- Care should be taken to use accurate thermophysical properties and the phase envelope should be plotted to avoid any operating problem.
Figure 5. Variation of the pipeline temperature profile with the N2 impurities and U=0.5 Btu/hr-˚F-ft2 (2.839 W/m2-˚C)
Figure 6. Variation of the pipeline pressure profile with the N2 impurities and U=0.5 Btu/hr-˚F-ft2 (2.839 W/m2-˚C)
To learn more about similar cases and how to minimize operational problems, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), P81 (CO2 Surface Facilities), and PF4 (Oil Production and Processing Facilities) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- Bothamley, M.E. and Moshfeghian, M., “Variation of properties in the dese phase region; Part 1 – Pure compounds,” TOTM, http://www.jmcampbell.com/tip-of-the-month/2009/12/variation-of-properties-in-the-dense-phase-region-part-1-pure-compounds/, Dec 2009.
- Moshfeghian, M., ”Transportation of CO2 in the Dense Phase,” TOTM, http://www.jmcampbell.com/tip-of-the-month/2012/02/ , Feb 2012
- Moshfeghian, M., ”Transportation of CO2 in the Dense Phase,” TOTM, http://www.jmcampbell.com/tip-of-the-month/2012/01/, Jan 2012
- Peng, D. Y., and Robinson, D. B., Ind. Eng. Chem. Fundam., Vol. 15, p. 59, 1976.
ProMax 3.2, Bryan Research and Engineering

Very good submit. My partner and i examine something more difficult upon different sites each day. It genuinely ought to always be exciting to find out articles from various writers as well as observe a little another thing at their store. I would select make use of a few together with the written content on my website whether or not you do not thoughts. Natually I’ll supply you with a website link as part of your internet website. Many thanks for sharing.
There is no more.
hello there and thank you for your information – I’ve definitely picked up something new from right here.
I did however expertise some technical points using this website, since I experienced to reload the web site lots of times previous to I could get it to load correctly.
I had been wondering if your hosting is OK? Not that I’m complaining, but slow loading instances times will often affect your placement in google
and could damage your high-quality score if ads and marketing with Adwords.
Well I am adding this RSS to my email and can look out for much more of your respective intriguing content.
Make sure you update this again very soon.
my page; norton 360 2014
What i do not understood is in fact how you are not actually a lot more smartly-liked than you might be now.
You are so intelligent. You know therefore significantly in terms of this topic, made me in my opinion believe it from
so many numerous angles. Its like women and men are not fascinated unless it is something to
do with Lady gaga! Your own stuffs outstanding. At all times take care of
it up!
Nice weblog my! possibly your site numerous up fast! precisely web host are additionally you the persue of? Cthe very I have used your associate hyperlink to your put? i started desire this fact website loaded up as quickly as yours lol
I’ve recently started a blog, the information you offer on this website has helped me greatly. Thank you for all of your time & work.
I am really loving the theme/design of your
site. Do you ever run into any browser compatibility issues?
A number of my blog readers have complained about my blog not working correctly in Explorer but looks great in Safari.
Do you have any suggestions to help fix this problem?
Check out my webpage: personalized home decor
[Geraldo]
You have lots of useful pointers on this site. This is a well composed article that I have bookmarked for future reading. Have a fun.