In the June 2012 Tip of the Month (TOTM), we evaluated the accuracy of a recently published model by Mamrosh et al. [1] against experimental data for CO2 and H2S solubility in triethylene glycol (TEG) solution. Based on this model, we reproduced several diagrams that can be used quickly to determine the absorption of these acid gases in TEG solution. In this TOTM, we have used the same solubility model and produced more diagrams in a different format covering a wide range of operating conditions. The advantage of presenting diagrams in this new format is that less number of diagrams is needed. These diagrams are presented in terms of constant acid gas partial pressure for varying pressure and temperature and constant TEG concentration of 100 and 95 weight percent. A sample calculation showing the application of these diagrams is also provided and the result is compared with ProMax.
Predicted absorption levels for acid gases can be as high as about 10 SCF/gallon (75 SCM/m3) of TEG solution and depends on temperature, pressure, acid gas concentration in the vapor phase and TEG concentration. As shown in the June 2012 TOTM, the absorption of acid gases increases with TEG purity. The solution of acid gases in TEG solution lowers its pH and enhances corrosion. In addition, one of the main issues is dealing with the H2S that comes off the TEG flash separator and the still regenerator. This is a problem if vented (bad smell & toxic) and can be a significant source of emissions (SO2) if burned.
Mamrosh-Fisher-Matthews Solubility Model:
Recently, Mamrosh et al. [1] presented the following correlation based on the experimental data to estimate solubility of CO2 and H2S in TEG solution.
Wt%TEG is the weight % of TEG in liquid
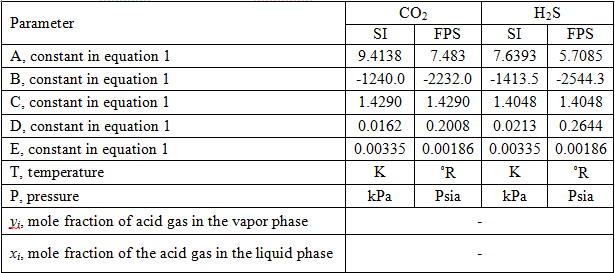
The values of the A, B, C, D, and E parameters for international (SI) and engineering field (FPS) units are given in Table 1. For details of the calculation procedure and a sample calculation refer to reference [1].
Table 1. Parameters for Mamrosh et al. model [2]
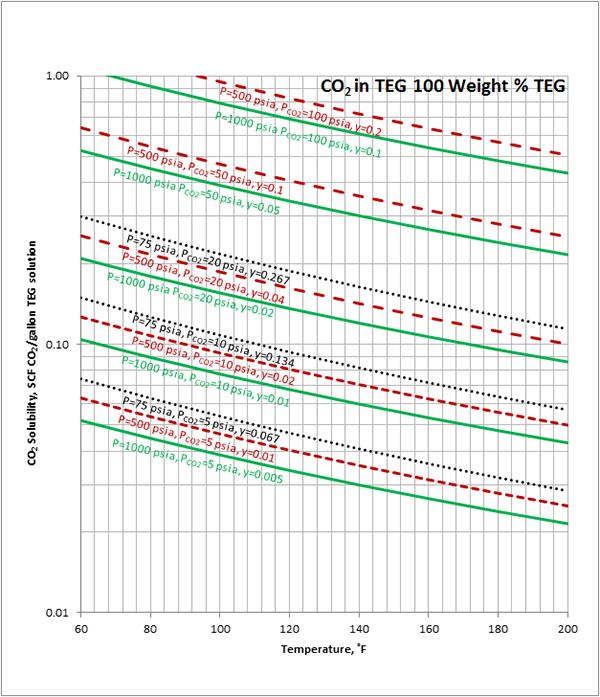
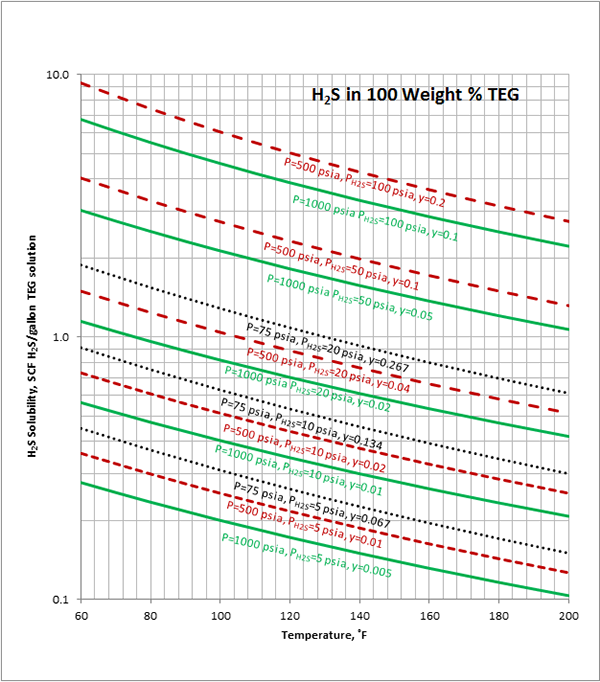
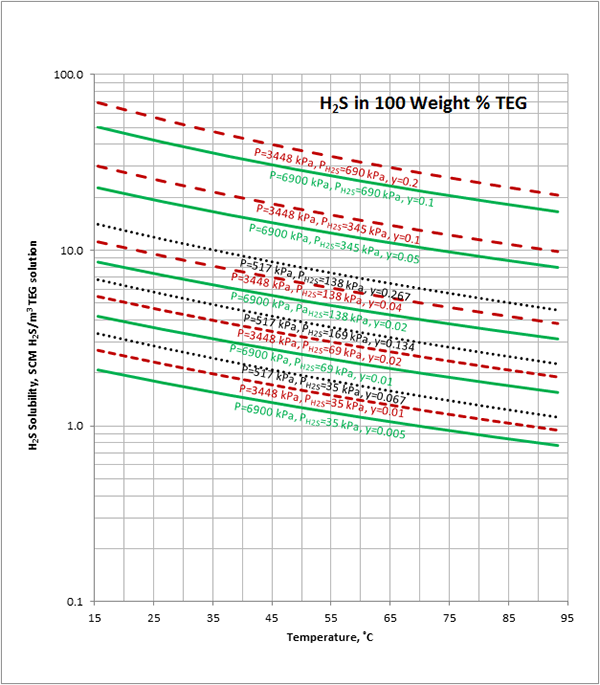
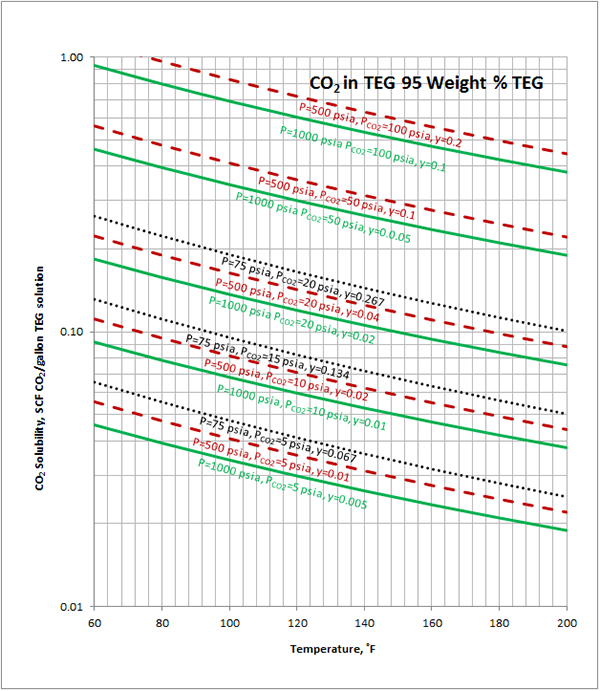
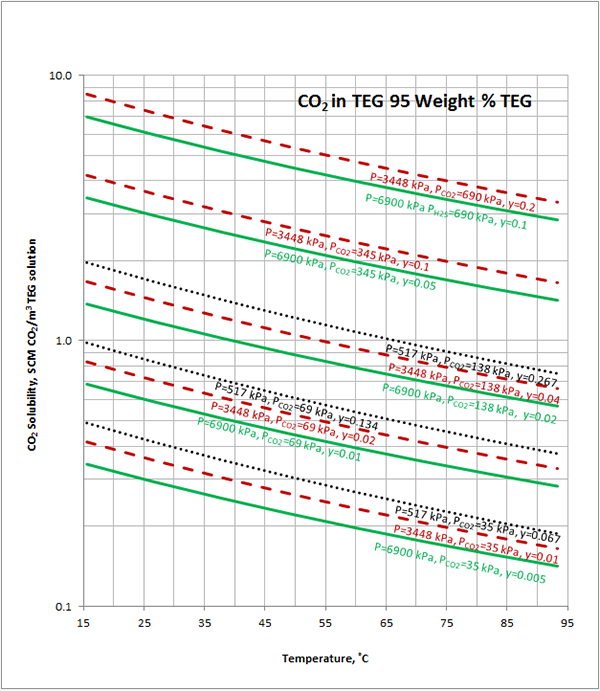
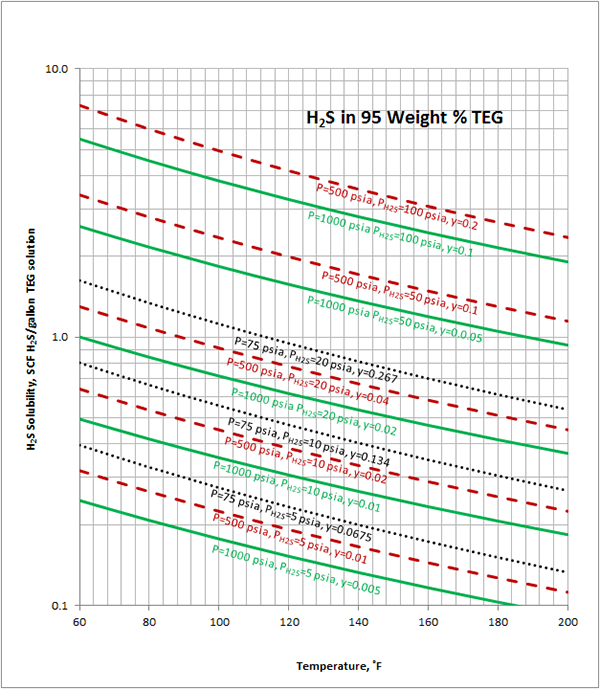
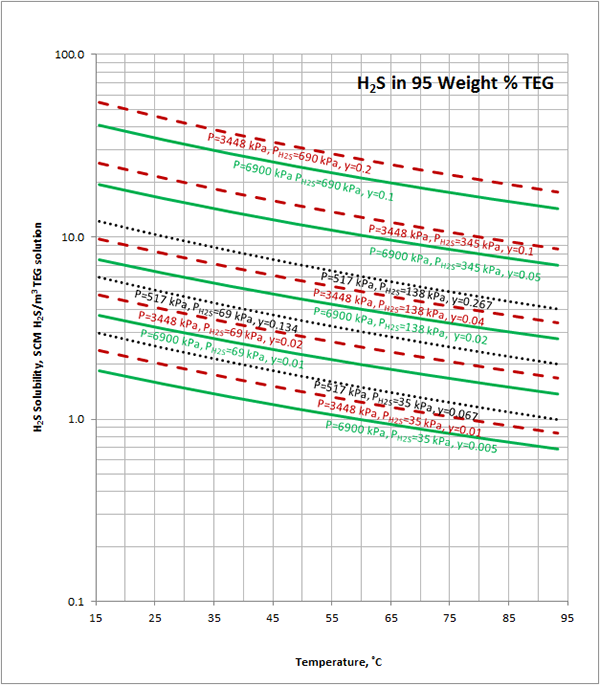
Case Study:In Figures 1 through 4 we have reproduced the CO2 and H2S solubility (on volumetric basis of SCF/gallon of TEG solution or SCM/m3 of TEG solution) for TEG concentration of 100 and 95 weight % for pressures of 1000 and 500 (6897 and 3448 kPa) representing contactor pressure, and 75 psia (517 kPa) representing the flash separator in a typical TEG dehydration unit. In each of these diagrams the solubility is presented as a function of temperature, acid gas partial pressure (mole %) in the gas phase based on the model proposed by Mamrosh et al. [1]. These figures are reproduced in the field or Engineering (FPS) and SI (International) systems of units. They can be quickly used to estimate acid gas absorption by TEG solution. In addition, a sample application of these diagrams is presented in the following section.
The volumetric feed flow rate to a TEG dehydration plant containing 1 mole % H2S is 200 MMSCFD (5.6634×106 SCMD). How many SCFD (SCMD) of H2S are released from the flash separator and regeneration column? The rich TEG concentration is 95 weight % and TEG circulation rate is 27 gallon/min (6.13 m3/h). Assume the contactor operates 100 ˚F (37.8 ˚C) and 1000 psia (6895 kPa). The flash drum operates at 75 psia (517 kPa) and 113 ˚F (45 ˚C) and there is about 6.7 mole % H2S in the flashed gas.
FPS Solution:
H2S partial pressure in feed gas = (0.01)(1000 psia) = 10 psia
From Figure 4 at T=100 ˚F, P = 1000 psia, H2S Partial Pressure= 10 psia,
0.36 SCF H2S/gallon of TEG is absorbed.
H2S partial pressure in flashed gas = (0.067)(75 psia) = 5 psia
From Figure 4 (FPS) at T=113 ˚F, P=75 psia, H2S Partial Pressure= 5 psia,
0.25 SCF H2S/gallon of TEG is absorbed.
H2S released with flashed gas = (27 gallon/min)(0.36-0.25)(SCF H2S/gallon TEG) = 2.97 SCF H2S/min = 4.28 MSCFD
H2S released in regenerator = (27 gallon/min)(0.25)(SCF H2S/gallon TEG) = 6.75 SCF H2S/min = 9.72 MSCFD
Total H2S released = 4.28 + 9.72 =13.997 MSCFD ≈ 14 MSCFD
H2S in Feed gas = (0.01)(200 000 MSCFD)=2000 MSCFD
Fraction of H2S absorbed = 100(14)/2000= 0.7 %
SI Solution:
H2S partial pressure in feed gas = (0.01)(6895 kPa) = 69 kPa
From Figure 4 (SI) at T=37.8 ˚C, P=6895 kPa, H2S Partial Pressure= 69 kPa,
2.7 SCM H2S/m3 of TEG is absorbed.
H2S partial pressure in flashed gas = (0.067)(517 kPa) = 35 kPa
From Figure 4 at T=45 ˚C, P=517 kPa, H2S Partial Pressure= 35 kPa,
1.9 SCM H2S/m3 of TEG is absorbed.
H2S released with flashed gas = (6.13 m3/h)(2.7-1.9)( SCM H2S/m3 TEG) = 4.904 SCM H2S/h = 117.7 SCM/d
H2S released in regenerator = (6.13 m3/h)(1.9)( SCM H2S/m3 TEG) = 11.647 SCM H2S/h = 279.5 SCM/d
Total H2S released= 117.7 + 279.5 =397.2 SCMD ≈ 400 SCMD
H2S in Feed gas= (0.01)( 5.6634×106 SCMD)=56 634 SCMD
Fraction of H2S absorbed = 100(400)/56 634= 0.7 %
We performed a rigorous simulation of a similar case as the above case study by ProMax [2] and the fraction of H2S absorbed turned out to be 0.78 %.
Conclusions:
In continuation of the June 2012 TOTM and to reduce the number of diagrams, we have produced several acid gas solubility diagrams in a different format that can be used quickly to determine the amount of acid gas release in the flash separator and from the regenerator column of a TEG dehydration unit. These diagrams (Figures 1-4) are based on the model developed by Mamrosh et al. [1] and are in the field (FPS) and SI systems of units and cover a wide range of operating conditions. For a case study, we have presented a sample calculation for estimation of H2S released with the flashed gas off the separator and from the overhead of the regenerator column. The results obtained for this case study compares well with those obtained from rigorous simulation using ProMax [2] software.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), G5 (Gas Conditioning and Processing-Special), PF81 (CO2 Surface Facilities), and PF4 (Oil Production and Processing Facilities) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- Mamrosh, D., Fisher, K. and J. Matthews, “Preparing solubility data for use by the gas processing industry: Updating Key Resources,” Presented at 91st Gas Processors Association National Convention, New Orleans, Louisiana, USA, April 15-18, 2012.
- ProMax 3.2, Bryan Research and Engineering, Inc., Bryan, Texas, 2011.
Figure 1 (FPS). Estimated CO2 solubility in 100 weight % TEG solution as a function of temperature, CO2 mole % (partial pressure) in vapor phase and pressure
Figure 1 (SI). Estimated CO2 solubility in 100 weight % TEG solution as a function of temperature, CO2 mole % (partial pressure) in vapor phase and pressure
Figure 2 (FPS). Estimated H2S solubility in 100 weight % TEG solution as a function of temperature, H2S mole % (partial pressure) in vapor phase and pressure
Figure 2 (SI). Estimated H2S solubility in 100 weight % TEG solution as a function of temperature, H2S mole % (partial pressure) in vapor phase and pressure
Figure 3 (FPS). Estimated CO2 solubility in 95 weight % TEG solution as a function of temperature, CO2 mole % (partial pressure) in vapor phase and pressure
Figure 3 (SI). Estimated CO2 solubility in 95 weight % TEG solution as a function of temperature, CO2 mole % (partial pressure) in vapor phase and pressure
Figure 4 (FPS). Estimated H2S solubility in 95 weight % TEG solution as a function of temperature, H2S mole % (partial pressure) in vapor phase and pressure
Figure 4 (SI). Estimated H2S solubility in 95 weight % TEG solution as a function of temperature, H2S mole % (partial pressure) in vapor phase and pressure

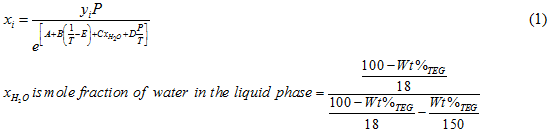
THANKS FOR YOUR UPDATE INFORMATION.KINDLY NOTIFY ME ANY NEW POST BY EMAIL.
YOURS
ARTHUR ODIBO
This excellent website certainly has all the information and facts I needed concerning this subject and didn’t know
who to ask.
Thank you for hosting this festival. It will be cool to look through everyone’s entries.