In gas dehydration service, triethylene glycol (TEG) will absorb limited quantities of BTEX (benzene, toluene, ethylbenzene, and xylene) and acid gases such as carbon dioxide (CO2) and hydrogen sulfide (H2S) from the gas. Predicted absorption levels for acid gases can be as high as about 10 SCF/gallon (75 SCM/m3) of TEG solution and depends on temperature, pressure, acid gas concentration in the vapor phase and TEG concentration. Figure 18.16 in reference [1] shows the solubility of H2S in TEG at various H2S partial pressures. This is true absorption that takes place in the absorber and corresponds to typical actual plant data. Figure 18.17 also in reference [1] shows solubility of CO2 in a 96.5 weight percent TEG solution. The absorption of acid gases increases with TEG purity. The solution of acid gases in TEG solution lowers its pH and enhances corrosion. In addition, one of the main issues is dealing with the H2S that comes off the still regenerator. This is a problem if vented (bad smell & poisonous) and can be a significant source of emissions (SO2) if burned.
In the June 2011 tip of the month (TOTM), we presented diagrams for quick estimation of absorption of BTEX in the glycol dehydration systems using the experimental vapor-liquid equilibrium data. The objective of this TOTM is to reproduce similar diagrams covering wide ranges of operating conditions. First we demonstrate the accuracy of a recent model proposed by Mamrosh et al. [2] against Gas Processors Association experimental data and then reproduce some of their recommended diagrams for approximate and quick estimation of acid gas absorption in TEG solution.
Mamrosh-Fisher-Matthews Solubility Model:
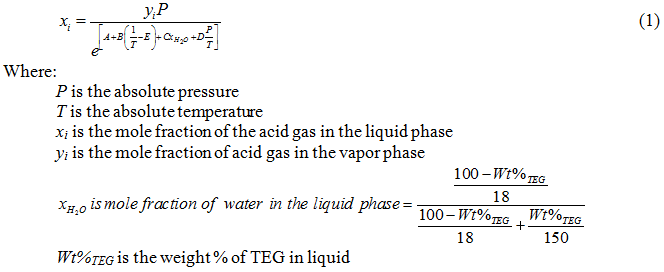
Recently, Mamrosh et al. [2] presented the following correlation based on the experimental data to estimate solubility of CO2 and H2S in TEG solution.
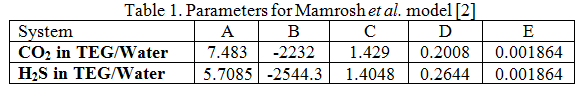
The values of the A, B, C, D, and E parameters are given in Table 1. For details of the calculation procedure and a sample calculation refer to reference [2].
Accuracy of Mamrosh-Fisher-Matthews Solubility Model:
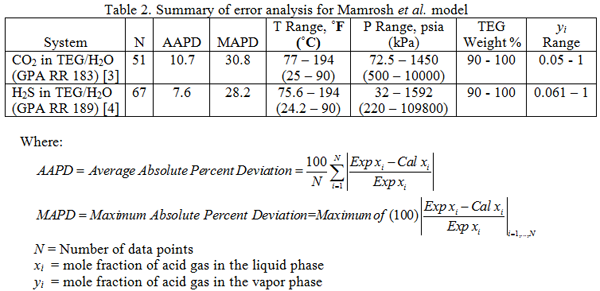
The accuracy of the Mamrosh et al. [2] model was evaluated against the experimental data of Gas Processor Association Research Reports RR 183 [3] and RR 189 [4] for CO2 and H2S solubility in TEG solution, respectively. The summary of our evaluation results is shown in Table 2.
It should be noted that for three cases of experimental data of H2S in TEG/H2O system, the absolute percent deviations were abnormally high (128, 260, and 319 %); therefore, they were eliminated from our analysis. Considering the error analysis shown in Table 2, the proposed model by Mamrosh et al. [2] has good accuracy for estimating solubility of acid gases in TEG/H2O solution. All experimental data reported in GPA RR 183 and RR 189 were collected at equilibrium. No consideration in the proposed model is given to the rate at which processes reach equilibrium.
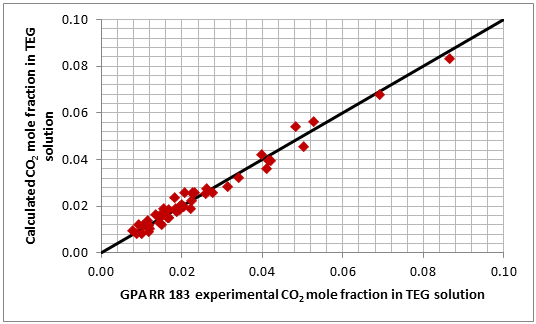
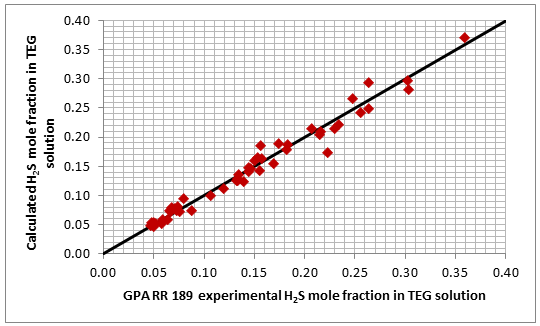
Figures 1 and 2 present a graphical comparison of the calculated CO2 and H2S solubility (mole fraction of acid gas in the liquid phase) with the experimental data of GPA RR 183 and GPA RR 189 for CO2 and H2S, respectively. Overall, good accuracy is observed for both systems in these two figures. The ranges of data are the same as those shown in Table 2.
Figure 1. Accuracy of the proposed model by Mamrosh et al. [2] for estimating CO2 solubility in TEG solution against GPA RR 183 experimental data [3]
Figure 2. Accuracy of the proposed model by Mamrosh et al. [2] for estimating H2S solubility in TEG solution against GPA RR 189 experimental data [4]
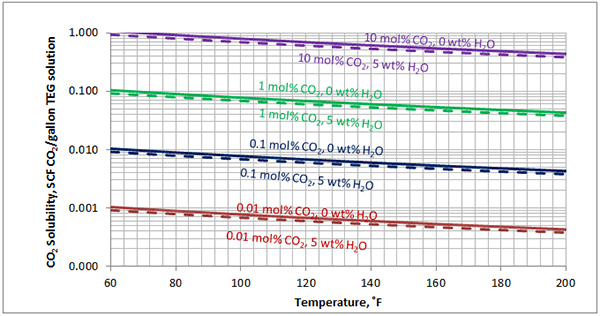
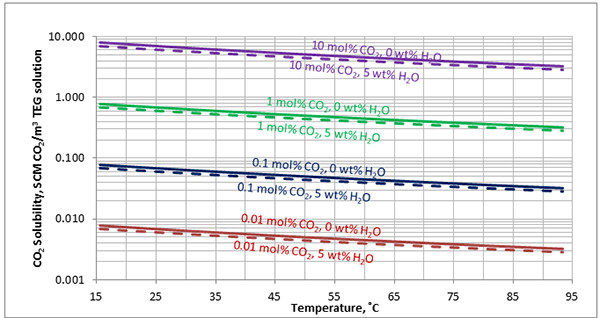
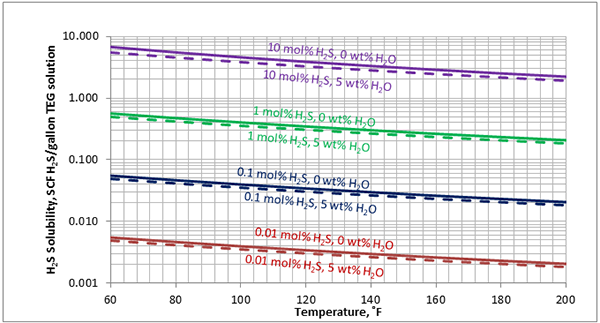
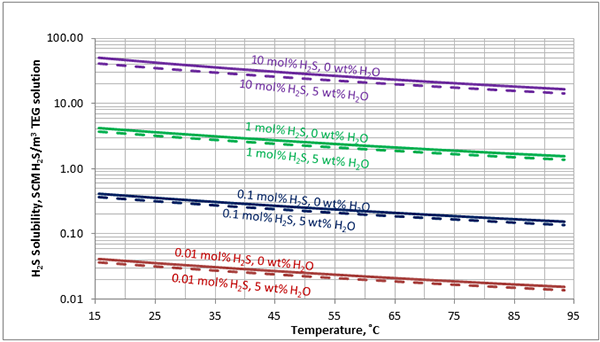
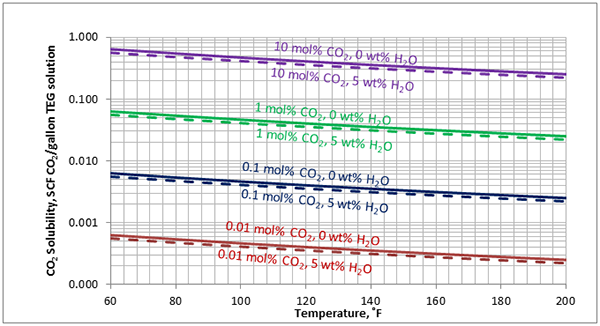
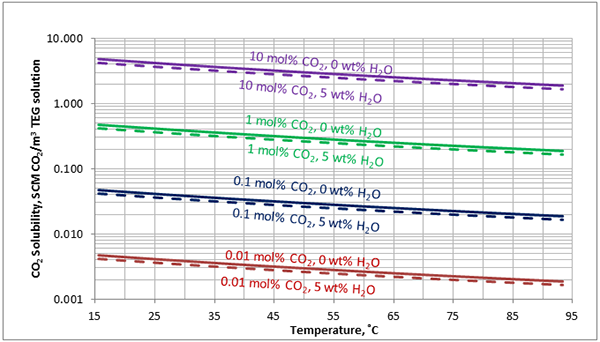
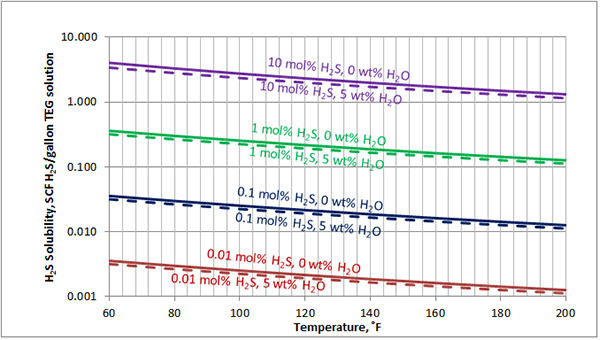
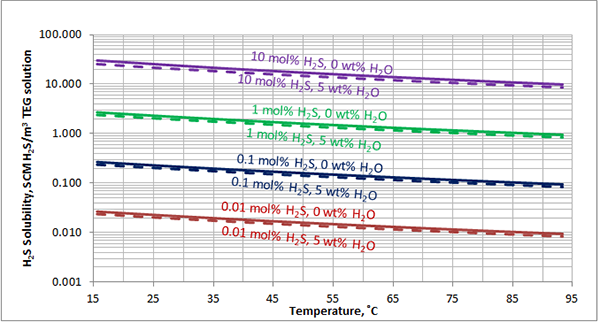
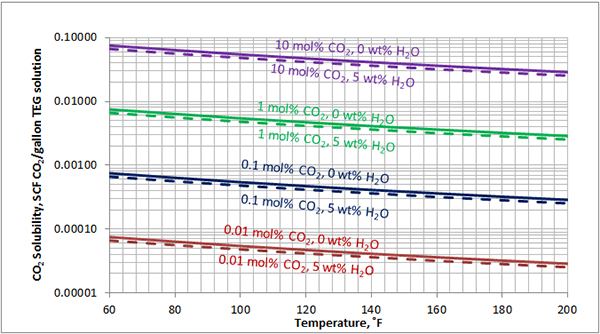
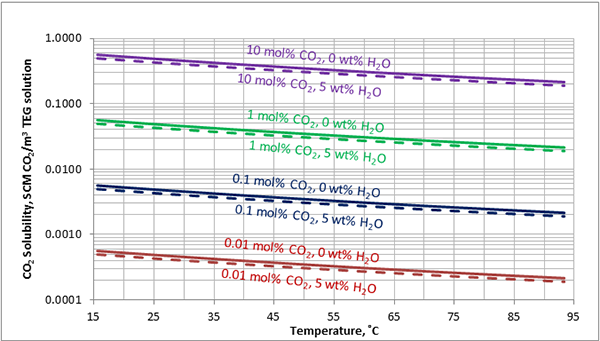
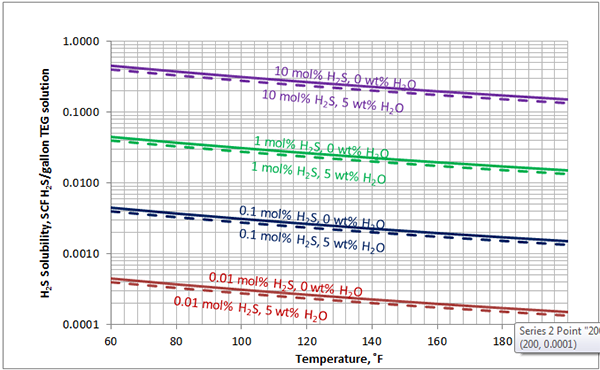
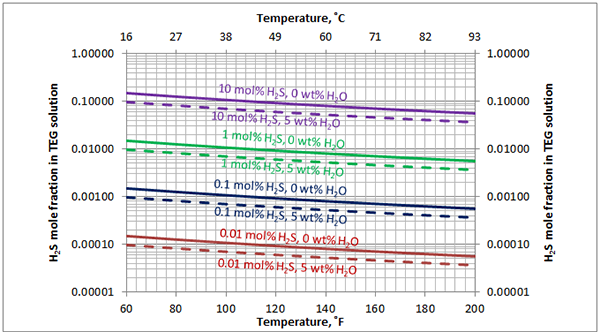
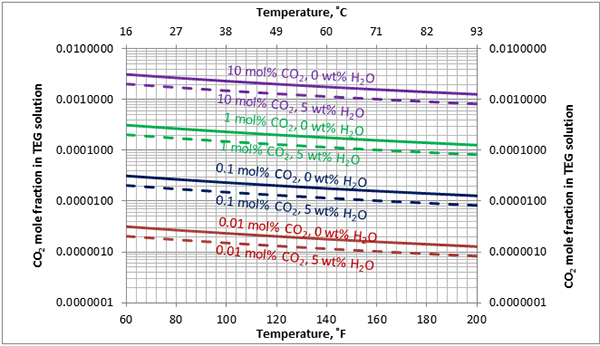
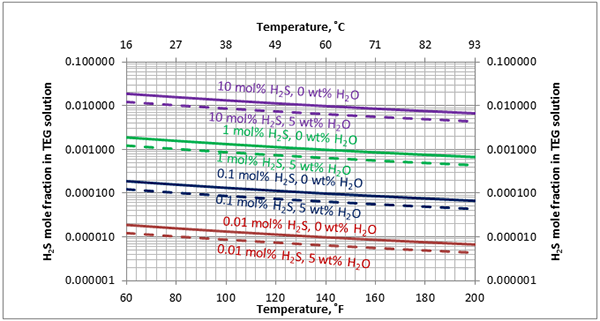
In Figures 3 through and 8 we have reproduced the CO2 and H2S solubility (on volumetric basis of SCF/gallon of TEG solution or SCM/m3 of TEG solution) for pressures of 1000 and 500 (6897 and 3448 kPa) representing contactor pressure, and 50 psia (345 kPa) representing the flash separator in a typical TEG dehydration unit. In each of these diagrams the solubility is presented as a function of temperature, acid gas mole % in the gas phase, and H2O weight % in TEG solution based on the model proposed by Mamrosh et al. [2]. These figures are reproduced in the field or Engineering (FPS) and SI (International) systems of units. They can be quickly used to estimate acid gas absorption by TEG solution. In addition, Figures A1 through A6 in Appendix A present solubility of acid gases in terms mole fraction instead of volume basis.
Conclusions:
We have performed an independent evaluation of a recently developed model by Mamrosh et al. [2] for estimation of acid gas absorption by TEG solution while dehydrating natural gas. Our evaluation was based on the experimentally measured data reported in the GPA RR 183 [3] and GPA RR 189 [4]. All experimental data reported in GPA RR 183 and RR 189 were collected at equilibrium. No consideration in the proposed model is given to the rate at which processes reach equilibrium.
The analysis of Figures 1 and 2 and Table 2 indicates, that even though the Mamrosh et al. [2] model is simple and easy to use, it is relatively accurate for estimation purposes. It also covers a wide range of operating conditions. Based on this model we have reproduced Figures 3 through 8 in the field and SI systems of units that can be used to estimate the absorption of CO2 and H2S in TEG solution during gas dehydration. The analysis of Figures 3 through 8 also indicates that at the same conditions, the solubility of H2S is almost 5 times greater than that of CO2. In addition, it can be concluded that the absorption of acid gases increase as:
- Pressure increases
- Temperature decreases
- Acid gas concentration in gas phase increases
- TEG concentration in liquid phase increases
- TEG solution circulation rate increases
To learn more about similar cases and how to minimize operational problems, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), PF81 (CO2 Surface Facilities), and PF4 (Oil Production and Processing Facilities) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Figure 3 (FPS). Estimated solubility CO2 in TEG solution at 1000 psia as a function of temperature, CO2 mole % in vapor phase and water weight %
Figure 3 (SI). Estimated solubility CO2 in TEG solution at 6897 kPa as a function of temperature, CO2 mole % in vapor phase and water weight %
Figure 4 (FPS). Estimated solubility H2S in TEG solution at 1000 psia as a function of temperature, H2S mole % in vapor phase and water weight %
Figure 4 (SI). Estimated solubility H2S in TEG solution at 6897 kPa as a function of temperature, H2S mole % in vapor phase and water weight %
Figure 5 (FPS). Estimated solubility CO2 in TEG solution at 500 psia as a function of temperature, CO2 mole % in vapor phase and water weight %
Figure 5 (SI). Estimated solubility CO2 in TEG solution at 3448 kPa psia as a function of temperature, CO2 mole % in vapor phase and water weight %
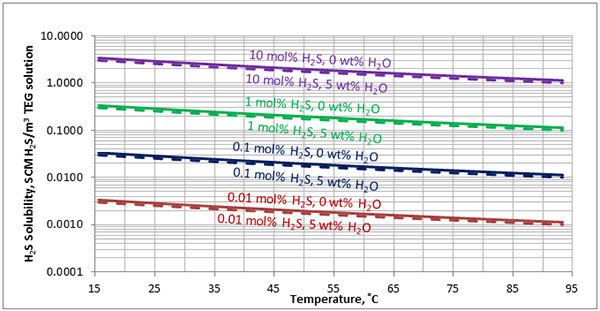
Figure 6 (FPS). Estimated solubility H2S in TEG solution at 500 psia as a function of temperature, H2S mole % in vapor phase and water weight %
Figure 6 (SI). Estimated solubility H2S in TEG solution at 3448 kPa as a function of temperature, H2S mole % in vapor phase and water weight %
Figure 7 (FPS). Estimated solubility CO2 in TEG solution at 50 psia as a function of temperature, CO2 mole % in vapor phase and water weight %
Figure 7 (SI). Estimated solubility CO2 in TEG solution at 345 kPa psia as a function of temperature, CO2 mole % in vapor phase and water weight %
Figure 8 (FPS). Estimated solubility H2S in TEG solution at 50 psia as a function of temperature, H2S mole % in vapor phase and water weight %
Figure 8 (SI). Estimated solubility H2S in TEG solution at 345 kPa as a function of temperature, H2S mole % in vapor phase and water weight %
Reference:
- Campbell, J. M. “Gas conditioning and processing, Volume 2: The Equipment Modules,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.
- Mamrosh, D., Fisher, K. and J. Matthews, “Preparing solubility data for use by the gas processing industry: Updating Key Resources,” Presented at 91st Gas Processors Association National Convention, New Orleans, Louisiana, USA, April 15-18, 2012.
- Davis, P.M., et al.; “The Impact of Sulfur Species on Glycol Dehydration – A Study of the Solubility of Certain Gases and Gas Mixtures in Glycol Solutions at Elevated Pressures and Temperatures, Revised RR Draft for Phase I: CO2/CH4/EG/TEG;” GPA Research Report, RR-183; Gas Processors Association., Tulsa Oklahoma, USA, 2002.
- Marriott, R.A., et al.; “The impact of Sulfur Species on Glycol Dehydration – A Study of the Solubility of Certain Gases and Gas Mixtures in Glycol Solutions at Elevated Pressures and Temperatures, VLE Data for the H2S/CH4/EG/H2O System and the H2S/CH4/TEG/H2O System,” GPA Research Report, RR-189; Gas Processors Association., Tulsa Oklahoma, USA, 2005.
Appendix A
Additional solubility Diagrams
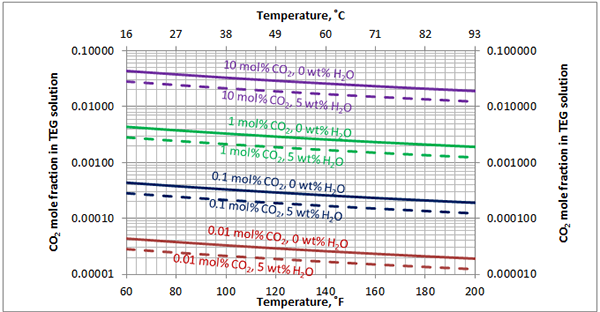
Figure A1. Estimated solubility CO2 in TEG solution at 1000 psia [6897 kPa] as a function of temperature, CO2 mole % in vapor phase and water weight %
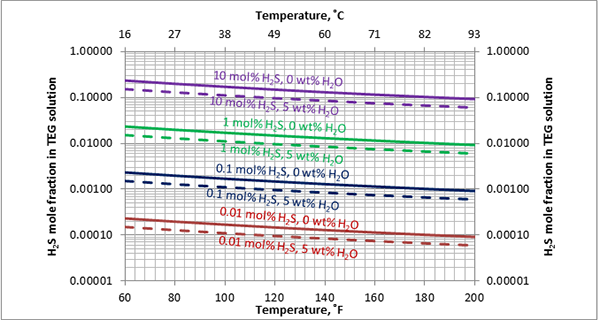
Figure A2. Estimated solubility H2S in TEG solution at 1000 psia [6897 kPa] as a function of temperature, H2S mole % in vapor phase and water weight %
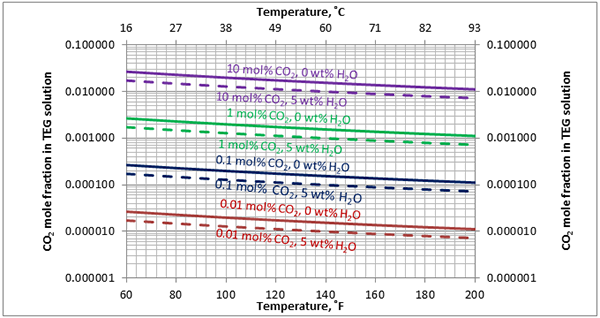
Figure A3. Estimated solubility CO2 in TEG solution at 500 psia [3448 kPa] as a function of temperature, CO2 mole % in vapor phase and water weight %
Figure A4. Estimated solubility H2S in TEG solution at 500 psia [3448 kPa] as a function of temperature, H2S mole % in vapor phase and water weight %
Figure A5. Estimated solubility CO2 in TEG solution at 50 psia [345 kPa] as a function of temperature, CO2 mole % in vapor phase and water weight %
Figure A6. Estimated solubility H2S in TEG solution at 50 psia [345 kPa] as a function of temperature, H2S mole % in vapor phase and water weight %

very good study , thanks very much , however i think there is a typo error in the conclusion as follows:
As per the presented graphs we can conclude that the absorption of acid gases increase as temperature decrease not increase, please verify and correct this typo error in the conclusion part of this study if needed
again thanks alot for TOTM we are really so interested on it
Thanks for your comment, Abdelrahman. This typo has been corrected.
Very informative and comphrensive. Do we have similar figures for low pressures at the Regenerator still where the pressure is close to atmospheric and the temperature is around 370 deg F ?
This is to determine how much of H2S / CO2 will be in the lean TEG solution AFTER Regenerator stripping i.e. how much of H2S / CO2 is “unavoidable” in the lean TEG solution.
Also, solubility data at the Regenerator conditions will help in doing a rough mass balance of the H2S and CO2 which comes out with the rich TEG, how much leaves via the Flash Drum / Regenerator and how much gets “recycled” back to the Contactor via the lean TEG stream.
good one
Very informative article, thanks. – R Hussain
Since the article was written was a follow up study of solubility at regenerator conditions prepared?
I had no idea that TEG would absorb limited quantities of BTEX and acid gasses! I can see how useful that would be as a way to remove unwanted aerosol compounds. I would imagine that finding a supplier of TEG to work with regularly would be a good way to keep your costs low as you lose it.
Dear Mahmood,
Would like to know is there a way we can calculate the solubility of oxygen in TEG at ambient condition ?
Or is it even possible that oxygen will dissolve into TEG when expose to ambient condition ?