Hydrocarbons are frequently produced with non-hydrocarbon impurities. The most common include water, carbon dioxide, hydrogen sulfide and nitrogen. We have already discussed water-hydrocarbon phase behavior in detail in the October and November 2007 Tips of the Month (TOTM). Since water has a low vapor pressure and is virtually immiscible in the hydrocarbon liquid phase, it does not have a significant effect on the shape of the hydrocarbon phase envelope except at high temperatures and low pressures.
The qualitative effect of CO2, H2S and N2 on the phase envelope of a rich gas or oil is shown in Figure 4.9 on page 100 in reference [1]. As shown in Figure 4.9 a and b, both CO2 and H2S lower the cricondenbar of the mixture. If sufficient quantities of the CO2 and H2S components are added to a reservoir fluid and the reservoir pressure is kept above the phase envelope, a single dense fluid phase exists. Although the actual mechanism is more complex, it is this solubility that is the primary driving force behind miscible flood enhanced oil recovery projects. NGL components such as ethane, propane and butane have a similar effect. With the increasing environmental concerns associated with acid gas (CO2 and/or H2S) injection into the reservoir and enhanced oil recovery, a good understanding of the impact on phase behavior is essential.
Nitrogen, on the other hand, raises the cricondenbar and decreases miscibility. It is sometimes used for pressure maintenance. There are also a few nitrogen miscible floods.
In this TOTM, we will study the impact of CO2, H2S and N2 on the phase behavior of different reservoir fluids such as black oil, volatile oil, and a rich gas. Computer simulated phase envelopes showing the quantitative effect are presented and discussed.
The Peng-Robinson (PR) [2] equation of state (EOS) option of ProMax [3] was used to perform all of the calculations in this study. In dealing with high content acid gases or nitrogen, care should be taken to verify the accuracy of an equation of state for handling these constituents. In general it is wise to assume the equations of state are inaccurate for modeling the thermodynamic properties and the phase behavior of systems containing high concentrations of non-hydrocarbon components like acid gases and nitrogen. Verification with experimental data is recommended before accepting results from equations of state.
Case Studies:
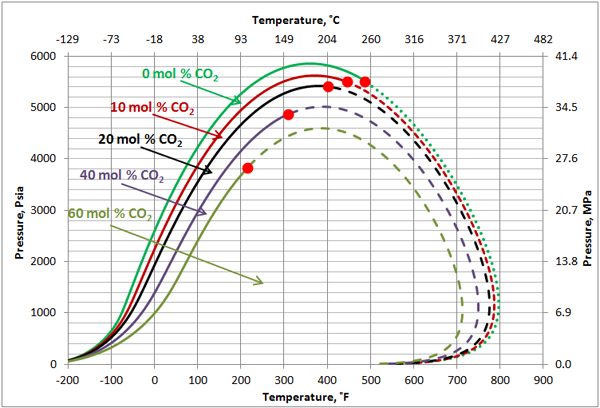
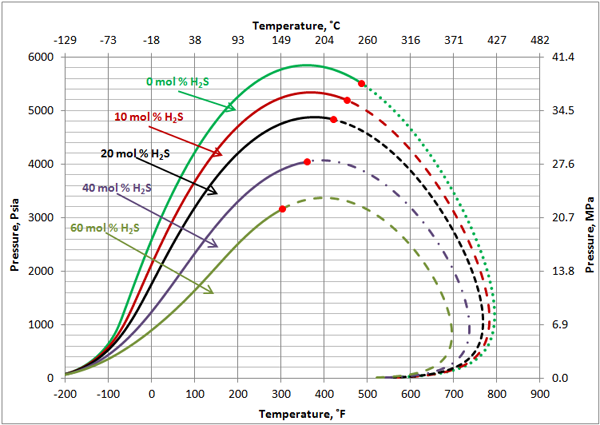
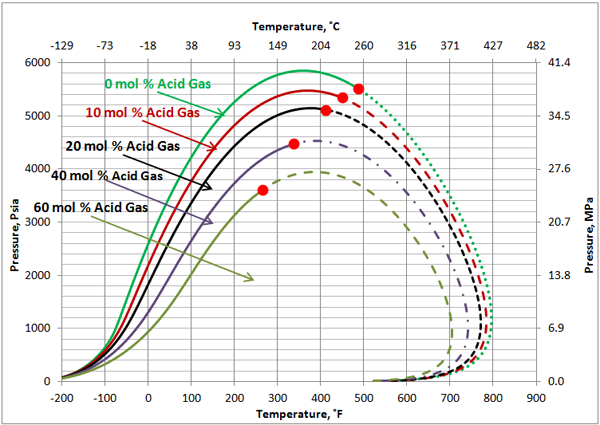
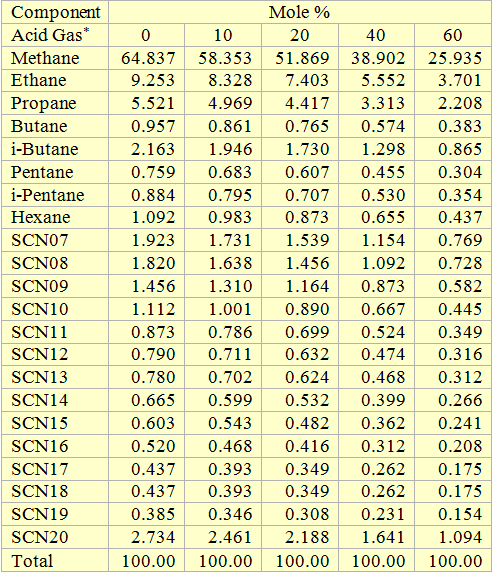
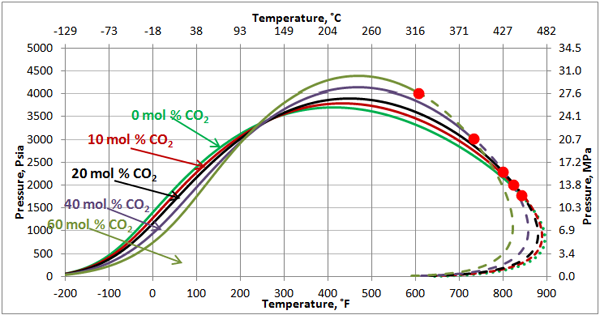
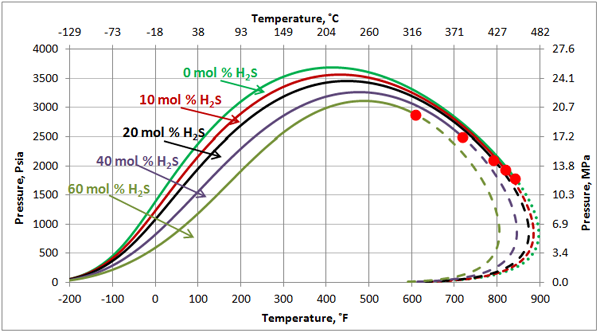
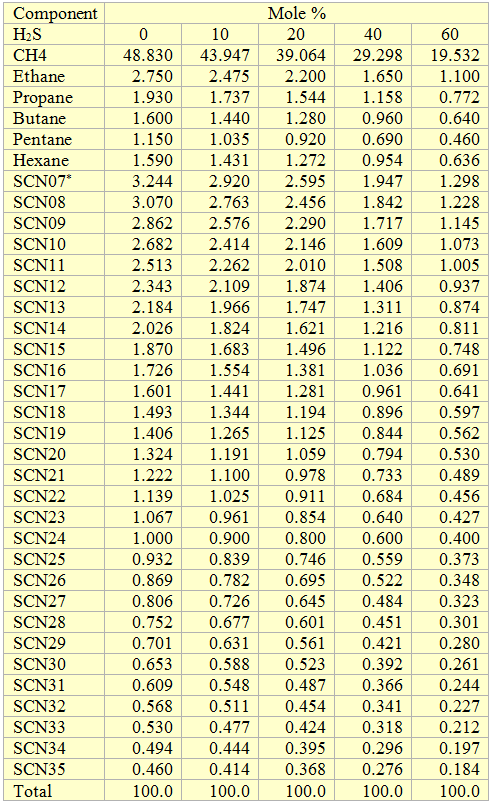
Volatile Oil: Figures 1, 2 and 3 present the impact of CO2 and H2S and their mixture on the phase behavior of a volatile oil. The compositions of the light oil and the acid gas used to generate these two figures are shown in Table 1. For the properties (average normal boiling point, molecular weight and relative density of single carbon number (SCN), see Table 3.3 on page 64 of reference [1]. Both CO2 and H2S lower the cricondenbar of the volatile oil. These quantitative behaviors agree well with the qualitative ones shown in Figure 4.9 a and b. Figure 3 presents the impact of equal molar mixtures of CO2 and H2S on the volatile oil phase envelope. The net effect is almost midway of the effect of CO2 and H2S. In all three figures, the critical point of mixture shifts considerably to the left. The cricondentherm point also shifts to the left as the concentration of acid gases increase. The net effect is enhancing miscibility, shrinkage of the two phase region and expanding the liquid phase region. These are all desirable for enhanced oil recovery.
Figure 1. The impact of CO2 concentration on the volatile oil phase envelope
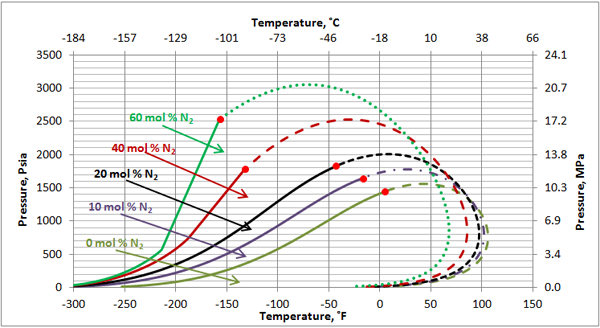
Rich Gas: The compositions of the rich gas and the non-hydrocarbons used to generate Figures 4, 5 and 6 are shown in Table 2. Figures 4, 5 and 6 present the impact of N2, CO2 and H2S on the phase behavior of the rich gas, respectively. As shown in Figure 4, N2 raises the cricondenbar of the rich gas. This quantitative behavior agrees well with the qualitative one shown in Figure 4.9 c. Nitrogen raises the cricondenbar, shifts the critical point to the left and decreases miscibility; therefore, it is best used for pressure maintenance. Miscibility can be attained only at very high pressures. Note for the case of 60 mole % in Figure 3, the bubble point curve and the critical point look abnormal which indicates that the equation of state and/or the binary interaction parameters used are incapable of handling high concentrations of N2
Figure 2. The impact of H2S concentration on the volatile oil phase envelope
Figure 3. The impact of acid gas (equal mole H2S and CO2) concentration on the volatile oil phase envelope.
Figure 5 presents the impact of CO2 concentration on the rich gas phase envelope. Like the case of the volatile oil, CO2 lowers the cricondenbar, shifts the cricondentherm to the right but shifts the critical point to the left.
Figure 6 presents the impact of H2S concentration on the rich gas phase envelope. Both the critical and cricondentherm points shift to the right as H2S increases but the cricondenbar does not lower as it did for CO2.
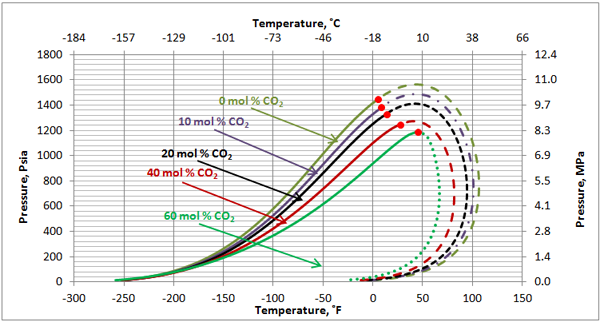
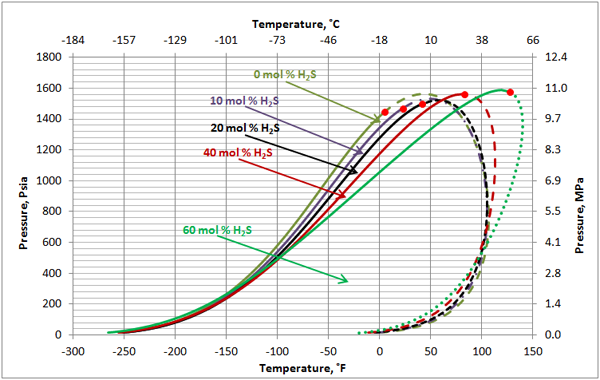
Black Oil: Figures 7 and 8 present the impact of CO2 and H2S on the phase behavior of black oil. The compositions of the black oil and the acid gas used to generate these two figures are shown in Table 3.
As shown in Figure 7, contrary to the case of the volatile oil, the cricondenbar raises as the CO2 content increases but both the critical and cricondentherm points shift to the left. Compared to Figure 1 for the volatile oil, the impact of CO2 on the black oil phase envelope is much less.
Table 1. Composition of the volatile oil used to generate Figures 1, 2, and 3.
* Acid Gas = H2S, CO2, or equal molar mixture of H2S, CO2.
The impact of H2S on this black oil is similar to its impact on the volatile oil (Figure 2). As shown in Figure 8, H2S lowers the cricondenbar of this black oil. The critical point shifts considerably to the left. The cricondentherm point also shifts to the left as the concentration of H2S increases. The net effect is enhancing miscibility, shrinkage of the two-phase region and expanding the liquid phase region. The impact of H2S on the black oil is less compared to the volatile oil shown in Figure 2.
Figure 4. The impact of N2 concentration on the rich gas phase envelope
Figure 5. The impact of CO2 concentration on the rich gas phase envelope
Figure 6. The impact of H2S concentration on the rich gas phase envelope
Table 2. Composition of the rich gas used to generate Figure 4, 5, and 6.
* Non-Hydrocarbons = N2, H2S, or CO2,.
Figure 7. The impact of CO2 concentration on the black oil phase envelope
Figure 8. The impact of H2S concentration on the black oil phase envelope
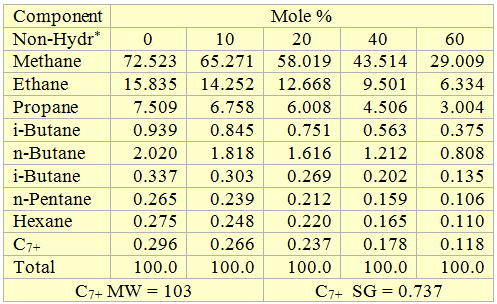
Table 3. Composition of the black oil used to generate Figures 7 and 8.
* For properties (average normal boiling point, molecular weight and relative density of single carbon number (SCN), see Table 3.3 page 64 of reference [1].
Conclusions:
The analysis of Figures 1 through 8 indicates that the impact of non-hydrocarbons on any reservoir fluids depends on the type/nature and composition of the reservoir fluid. The type of non-hydrocarbon as well as its concentration also plays an important role. The injection of acid gases into a reservoir fluid changes the phase behavior and the thermodynamic properties of the reservoir fluids. Even though not discussed in this TOTM, CO2 injection for the purpose of enhanced oil recovery may cause asphaltene deposition and blockage in the reservoir formation and the surface facilities. Depending on compositions, pressures and temperatures, much more complex phase behavior is possible. Multiple liquid phases (in addition to aqueous phase) and/or solids may be present.
It is important to use the right tools and an accurate equation of state within simulation software to generate the correct phase envelope. It is recommended to check the accuracy of the thermodynamic models against field/experimental data before generating any phase envelope. The equation of state should be tuned to match the laboratory measured vapor-liquid-equilibria data for a sample of the reservoir fluid before undertaking any practical study/decision. The results shown in this TOTM are specific to the cases studied and have not been validated with actual data. These results should be used only as a guideline.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), P81 (CO2 Surface Facilities), and PF4 (Oil Production and Processing Facilities) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- Campbell, J. M. “Gas conditioning and processing, Volume 1: Fundamentals,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.
- Peng, D. Y., and Robinson, D. B., Ind. Eng. Chem. Fundam., Vol. 15, p. 59, 1976.
- ProMax 3.2, Bryan Research and Engineering, Inc., Bryan, Texas, 2011.

very interesting topic,
I would add that in many cases water content must be considered as it can signicantly affect phase equilibria,
examples are H2O-CO2, H2O-H2S etc.
this is also true for other reservoir fluids.
While I agree that it is not easy to solve multiphase equilibria at high pressures for a mixture of hydrocarbons + water (i.e. std. EOS as Peng Robincon could not give accurate results for all fluids in the mixture) there are alternatives, for example for hydrocarbons + water there are the models developed by GERG and others.
Nowadays simulators have procedures to plot a multiphase (vapor-liquid-solid) phase envelope, these are powerful tools for understanding phase equilibria,
the same power is available in Excel or other Windows applications at lower costs with libraries,
see for example
http://www.prode.com/en/phaseenvelope.htm
The sentance “Note for the case of 60 mole % in Figure 3, the bubble point curve and the critical point look abnormal which indicates that the equation of state and/or the binary interaction parameters used are incapable of handling high concentrations of N2” from the article refers.
I think there is a typo error since there is no reference to N2 in Figure 3 – I think Fig 3 should read Fig 4.
Yes, you are correct, it should be Figure 4. Thanks.
A lot of the things you say is astonishingly precise and it makes me ponder why I hadn’t looked at this with this light previously. Your piece really did turn the light on for me as far as this particular subject matter goes. But there is 1 position I am not really too comfy with and whilst I try to reconcile that with the central idea of your issue, allow me observe what all the rest of the readers have to say.Well done.
This will be a great blog, might you be interested in doing an interview regarding how you developed it? If so e-mail me!