A phase envelope with hydrate and water dew point curves is an excellent tool to find what phase water is in at operating conditions, during start-up, during shut-down and during upsets. In the November 2007 Tip of the Month (TOTM), we discussed the phase behavior of water-sour natural gas mixtures. In this tip, we will extend our study on the sour natural gas hydrate formation phase behavior. Specifically, we will study the impact of H2S and CO2 on the formation of hydrate in natural gas.
The hydrate formation temperature of a gas depends on the system pressure and composition. There are several methods of calculating the hydrate formation conditions of natural gases. At equilibrium, the chemical potential of water in the hydrate phase is equal to that in each of the other coexisting phases. Parrish and Prausnitz [1] developed a thermodynamic model to describe this phenomenon, and later, the model was improved by Holder et al. [2]. These methods are suitable for calculations using a computer with equations of state. The details of hand calculation methods can be found in Chapter 6 of Volume 1 [3] of “Gas Conditioning and Processing” and Chapter 20 of GPSA DATA BOOK [4]. In this work we will use the Soave-Redlich-Kwong (SRK EoS) [5] in ProMax [6] software.
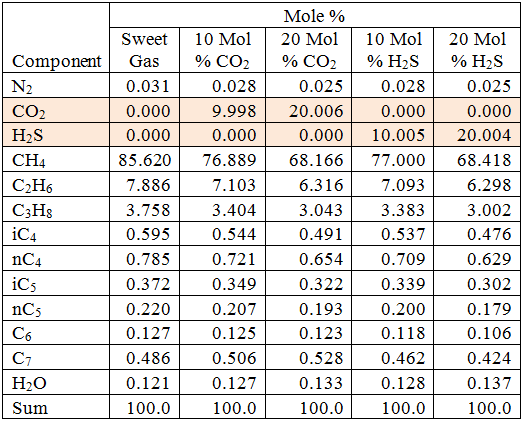
The compositions of the gas mixture studied in this study are shown in Table 1.
Table 1. Water-saturated compositions of gas mixtures studied
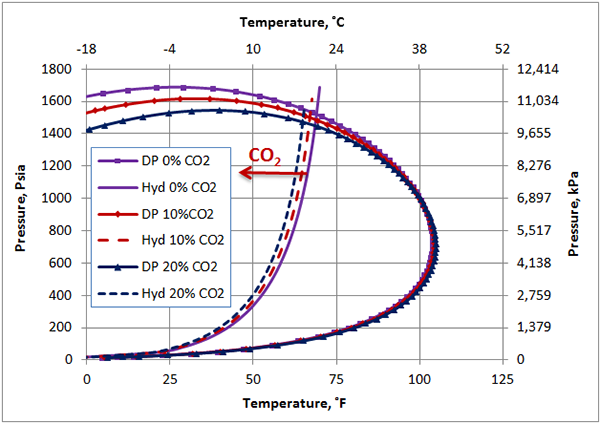
Figure 1 presents the calculated hydrate formation curve (solid curve) and the dew point portion of the phase envelope of a sweet natural gas (solid curve with the square). Figure 1 also presents the dew point and hydrate formation curves for the same gas mixture containing 10 and 20 mole % CO2. Figure 1 indicates that as the CO2 mole % increases from 0 to 20 mole %, the hydrate formation curves shift slightly to the left, depressing the hydrate formation temperature. Note that the points to the left and above the hydrate curves represent the hydrate formation region. From an operational point of view, this region should be avoided/prevented. This figure also indicates, as CO2 mole % increases, the cricondenbar decreases and the phase envelope shrinks.
Figure 1. The impact of CO2 on the hydrocarbon dew point and hydrate formation curve.
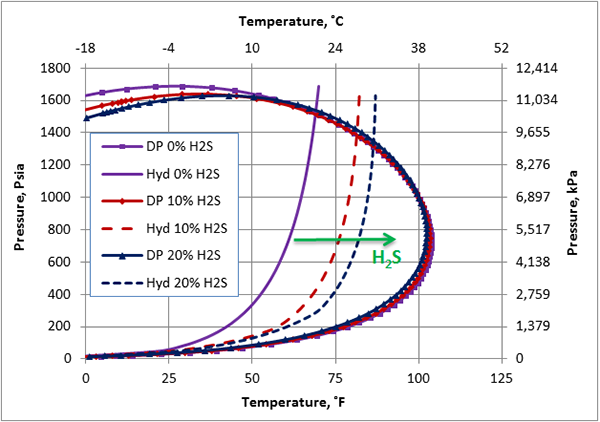
Similarly, Figure 2 presents the calculated hydrate formation curve (solid curve) and the dew point portion of phase envelope for the same sweet natural gas (solid curve with the square). Figure 2 also presents the dew point and hydrate formation curves for the same gas mixture containing 10 and 20 mole % H2S. Figure 2 indicates that as the H2S mole % increases from 0 to 20 mole %, the hydrate formation curves shift considerably to the right, promoting the hydrate formation temperature. This is opposite to the effect of CO2 and it is more pronounced. From an operational point of view, this is undesirable because H2S expands the hydrate formation region to the right. Note that the points to the right and below of the hydrate curve represent the hydrate-free region. Figure 2 also indicates, as H2S mole % increases, the cricondenbar decreases and the phase envelope shrinks. The shrinkage of the phase envelope is less than that of CO2.
Figure 2. The impact of H2S on the hydrocarbon dew point and hydrate formation curve.
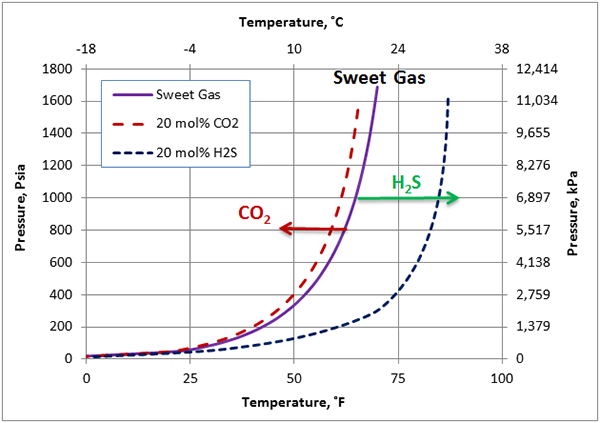
Figure 3 presents the calculated hydrate formation curves for a sweet gas, a sour gas with 20 mole % CO2 and a sour gas with 20 mole % H2S. This figure clearly indicates that the impact of H2S is much bigger than the CO2 impact; CO2 depresses (shifts to the left) the hydrate formation condition slightly but H2S promotes hydrate formation considerably. As an example, at 1000 psia (6900 kPa), CO2 reduces hydrate formation temperature for this gas by about 5.5˚F (3˚C) while, H2S increase hydrate formation temperature by about 20˚F (11.1˚C).
Conclusions:
The presence of CO2 and H2S in natural gas has an opposite impact on the hydrate formation condition. While the impact of CO2 is small, H2S has considerable impact on the hydrate formation condition. CO2 depresses hydrate formation (acts as hydrate inhibitor and shifts the hydrate curve to the left) while H2S shifts the hydrate curve to the right, promotes hydrate formation conditions, and may cause severe operational problems.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), P81 (CO2 Surface Facilities), and PF4 (Oil Production and Processing Facilities) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.com, or email us at consulting@jmcampbell.com.
By: Dr. Mahmood Moshfeghian
Figure 3. The opposite impact of CO2 and H2S on the hydrate formation curve.
Reference:
- Campbell, J.M., “Gas conditioning and Processing, Vol 1: The Basic Principles”, 8th Edition, Edited by R.A. Hubbard, John M. Campbell & Company, Norman, USA, 2001.
- Parrish, W.R., and J.M. Prausnitz, “Dissociation pressures of gas hydrates formed by gas mixtures,” Ind. Eng. Chem. Proc. Dev. 11: 26, 1972.
- Holder, G. D., Gorbin, G. and Papadopoulo, K.D, “Thermodynamic and molecular properties of gas hydrates from mixtures containing methane. argon, and krypton,” Ind. Eng. Chem. Fund. 19(3): 282, 1980.
- Gas Processors Suppliers Association; “ENGINEERING DATA BOOK” 13th Edition – FPS; Tulsa, Oklahoma, USA, 2012.
- G. Soave, Chem. Eng. Sci. 27, 1197-1203, 1972.
- ProMax 3.2, Bryan Research and Engineering, Inc, Bryan, Texas, 2012.

thanks
No comments , only question : why the TOTM of Jan-2013 does not issue yet?
regards
is this kind of exercises are part of G-4 Training course?
Yes. Of course, much more.
Dear Prof. Moshfeghian,
Are there any commercial software to simulate the process of CO2 capture by hydrate technology in a process?
Best Regards,
Your place is valueble for me. Thanks!…
[…] Moshfeghian, M. http://www.jmcampbell.com/tip-of-the-month/2012/12/sour-gas-hydrate-formation-phase-behavior/ […]
[…] Moshfeghian, M., http://www.jmcampbell.com/tip-of-the-month/2012/12/sour-gas-hydrate-formation-phase-behavior/ […]
[…] Moshfeghian, M.,http://www.jmcampbell.com/tip-of-the-month/2012/12/sour-gas-hydrate-formation-phase-behavior/ […]
[…] Moshfeghian, M.,http://www.jmcampbell.com/tip-of-the-month/2012/12/sour-gas-hydrate-formation-phase-behavior/ […]
Dear Prof,
Please kindly help me with Hydrate formation Reservoir Composition, this is to aid my project topic on Modelling hydrate formation using software pipesm.
Thanks.