The phase behavior of a light hydrocarbon compound like propane and water binary system is complicated for the following two reasons:
1. Very low mutual solubility in liquid phases
2. At lower temperatures, ice or hydrates is formed.
Reference [1] presents an excellent review of a propane – water system phase behavior. It gives an integral description of the phase behavior of a propane – water system from very high to very low pressures in the form of a series of consecutive isobaric temperature–composition diagrams. Special attention is given to equilibria involving hydrates.
Figure 1 illustrates the general behavior for a propane–water system [2]. As shown in this figure, for temperatures less than the freezing point of 32 °F (0 °C) both ice and hydrate are present above the hydrate formation (green) curve; only vapor and ice are below. Above the freezing point, hydrate is the only solid phase to the left of the hydrate forming (green) curve. Above the intersection of the vapor pressure (blue) and hydrate (green) curves, as pressure is increased the temperature will increase slowly.
For a pressure of 29 psia (0.2 MPa), the Hydrate+Ice region requires an overall composition of propane less than 5.56 mole %. The area marked as Hydrate+Ice also includes Vapor+Hydrate because there is actually a 3rd dimension to the plot – composition. For overall compositions of propane > 5.56 mole %, Vapor+Hydrate is encountered and as it cools from 50 °F (10°C) at 29 psia (0.2 MPa) to the propane saturation temperature, then it is Hydrate+Liquid propane.
During propane processing, transportation, and storage hydrate formation should be prevented. To calculate the hydrate formation temperature and the required amount of inhibitor (e.g. methanol) to inhibit hydrate formation, estimation of water content is required.
In this tip, we first will evaluate the accuracy of water content predicted by a process simulation software against limited measured experimental data. Secondly, the tip studies the effect of pressure and temperature on the propane water content in equilibrium with liquid water, ice, or hydrate phase. In addition, water content charts are presented for isobars of 14.7, 25, 50, 100, 150, and 200 psia (101.3, 172, 345, 699, 1034, 1379 kPa). For each isobar a temperature range of -60 °F to 200 °F (-51 °C to 104 °C) is covered.

Figure 1. Phase Behavior of Propane-Water System [2]
Evaluation of the Water Content Prediction Methods
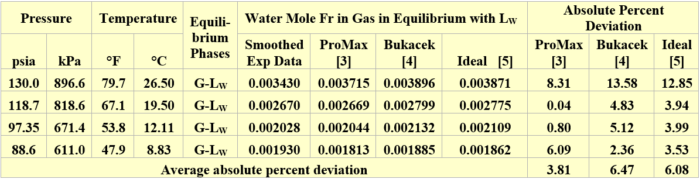
The performance of the ProMax simulation software [3], Bukacek correlation [4], and Raoult’s law (ideal) [5] for estimating the water content of propane vapor in equilibrium with liquid water (G–LW) was evaluated against GPA RR 132 experimental data [6]. A summary of propane vapor water content comparisons is presented in Table 1. For these set of pressures and temperatures, all three methods give good results. The SRK EOS (Soave-Redlich-Kwong equation of state) [7] with its ProMax default binary interaction parameters were used. The water content was predicted using the water saturator tool available in ProMax.
Table 1. Comparison of vapor propane water mole fractions by three methods against GPA-RR 132 [6] experimental data
Similarly, the performance of ProMax [3] for estimating the water content of liquid propane in equilibrium with liquid water or hydrate was evaluated against GPA RR 132 [6] experimental data. The SRK EOS (Soave-Redlich-Kwong equation of state) with its default binary interaction parameters was used in ProMax. A summary of liquid propane water content comparison results is presented in Table 2. Considering the very low solubility of water in liquid propane, the agreement between the predicted values and experimental data is very good.
For the liquid propane-liquid water equilibrium phases the ProMax water saturator tool was used to estimate the water content of the liquid propane phase. Note for the formation of liquid propane, the reported experimental pressure of Table 2 had to be increased. The adjusted pressure values are presented in parenthesis. Except for the first pressure, the adjustment of pressure is small. The 130 psia point for RR-132 must be an incorrect pressure as a liquid-liquid system will require a pressure greater than the vapor pressure of propane of ~ 144 psia [993 kPa] at the stated temperature.
For the liquid propane-hydrate equilibrium phases, one mole of pure propane stream was mixed with a pure water stream at the desired pressure. To determine the water content of the mixed stream, the solver tool of ProMax was used to adjust the pure water stream flow rate to form hydrate at the specified hydrate formation temperature. Table 2 indicates that even the liquid propane water contents are very low (0.00004 to 0.000388 mole fraction corresponding to 4 to 388 ppm by mole), the average absolute percent deviation is 10.2.
Table 2. Comparison of liquid propane water mole fractions in equilibrium with liquid water or hydrate by ProMax against the GPA-RR 132 [6] experimental data
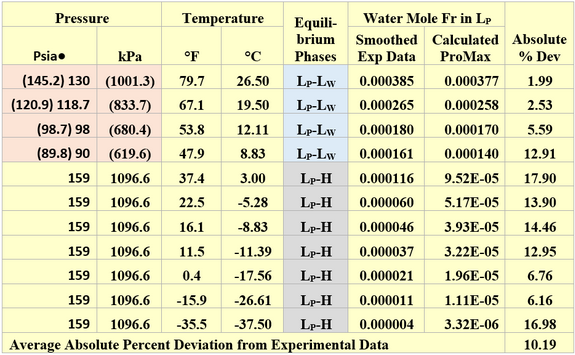
The numbers in parenthesis are the adjusted experimental values to form liquid-liquid phases.
Propane Water Content Charts
As illustrated in Figure 1, for the binary propane–water system, the coexistence of equilibrium phases depends on the system pressure and temperature as follows:
► Propane vapor phase in equilibrium with liquid water
► Propane vapor phase in equilibrium with ice or hydrate
► Propane liquid phase in equilibrium with liquid water
► Propane liquid phase in equilibrium with hydrate
Figure 2 illustrates the presence of these equilibrium phases as a function of temperature for the isobar of 14.7 psia (101.3 kPa).

Figure 2. Water content of vapor and liquid propane as a function of temperature at 14.7 psia (101.3 kPa)
As illustrated in Figure 1, the propane water content was estimated by the following procedures:
► For temperatures of 200 °F to about 27.5 °F (93 to ~ -2.5 °C), the propane vapor is in equilibrium with liquid water phase so the water saturator tool of ProMax was used.
► For temperatures of about 27.5 °F to -43.3 °F (~ -2.5 to -41.8 °C), the propane vapor was in equilibrium with ice or the hydrate phase, so one mole of pure propane stream was mixed with a pure water stream at a pressure of 14.7 psia (101.3 kPa). To determine the water content of the mixed stream, the solver tool in ProMax was used to adjust the pure water stream flow rate to form hydrate at the specified hydrate formation temperature.
► The propane vapor phase transition to the liquid phase takes place -43.3 °F (-41.8 °C)
► For temperatures of -43.3 °F to -60 °F (-41.8 to -51 °C), the propane liquid is in equilibrium with the hydrate phase, so one mole of pure propane stream was mixed with a pure water stream at a pressure of 14.7 psia (101.3 kPa). To determine the water content of the mixed stream, the solver tool in ProMax was used to adjust the pure water stream flow rate to form hydrate at the specified hydrate formation temperature.
Table 3 presents the three phase temperatures and the saturation temperatures of propane–water system estimated by ProMax.
Table 3. Three phase temperature and saturation temperature for six isobars
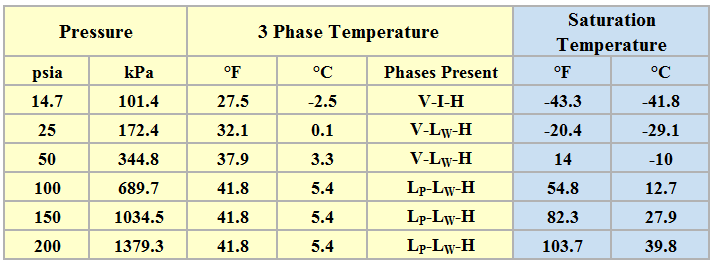
Phases: V = Vapor, I = Ice, H = Hydrate, LW = Liquid Water, and LP = Liquid Propane
Similarly, the water content charts of propane vapor and liquid phases were prepared for the other isobars and are presented in Figures 3-5. Note in Figures 3 and 5, due to the very small values, the liquid propane water contents for different isobars fall on the same curve.

Figure 3. Water content of propane as a function of temperature for six isobars

Figure 4. Water content of vapor propane as a function of temperature for several isobars

Figure 5. Water content of liquid propane as a function of temperature for several isobars
CONCLUSION
Estimating propane water content requires a good understanding of the phase behavior. The process simulation programs have several tools or procedures for estimating the water content. Which one should be used to give a correct answer? In addition to the selection of a suitable equation of state, the selection of the right tool or procedure at a given set of conditions is essential. The choice of a suitable tool changes as the conditions or the equilibrium phases change.
The presented propane water content charts can be used for facility type calculations and trouble shooting. It is a good practice to test the performance/accuracy of the selected tool against experimental data first. Obviously, for better understanding of propane–water phase behavior and improving the thermodynamic modeling more experimental data are needed.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing) and G5 (Practical Computer Simulation Applications in Gas Processing) courses.
By: Dr. Mahmood Moshfeghian

References
1. Harmens, A. and E.D. Sloan, “The phase Behavior of Propane – Water System: A Review,” The Canadian J of Chem Engr, Vol 68, Feb 1998.
2. Campbell, J.M., Gas Conditioning and Processing, Volume 1: The Basic Principles, 9th Edition, 2nd Printing, Editors Hubbard, R. and Snow–McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, 2014.
3. ProMax 4.0, Build 4.0.17179.0, Bryan Research and Engineering, Inc., Bryan, Texas, 2017.
4. Bukacek, R.F., “Equilibrium Moisture Content of Natural Gases” Research Bulletin IGT, Chicago, vol 8, 198-200, 1959.
5. Moshfeghian, M., “Ideal Water Content Correlation for Sweet Natural Gas,” PetroSkills TOTM, May 2018.
6. Song, K and R. Kobayashi, “Water content of ethane, propane, and their mixtures in equilibrium with water and hydrates,” Gas Processor Association Research Report (GPA RR 132), Tulsa, Oklahoma, 1991.
7. Soave, G., Chem. Eng. Sci. Vol. 27, No. 6, p. 1197, 1972.

[…] Moshfeghian, M., “Propane – Water Phase Behavior at Low to Moderate Pressures,” PetroSkills TOTM, Sep […]