There are a few computer tools designed specifically for modeling and analysis of complex multiphase systems such as PipePhase, PipeSim, OLGA, and etc [1]. Modeling and simulation of multiphase system, even under steady-state condition, is complex. In the June Tip of the Month (TOTM), we illustrated how the process simulation programs can be used to simulate a natural gas transmission pipeline. These programs are based on mechanistic models and laboratory developed correlations and rely on complex iterative algorithms to perform the tedious calculations.
However, for hand calculation, the Flanigan correlation (which is based on field data for gas dominated transmission pipelines) has been developed and can be used in relatively straight manual calculations. This correlation has proven useful even though it is relatively simple. The relationship between gas flow rate, diameter and pressure drop is represented by Panhandle A gas flow equation (which is based on the Basic Gas Flow Equation modified with field data). The basic equation is single phase flow for gas as is the Panhandle A Equation. The basic equation is derived from basic principles, while the Panhandle A and Flanagan Equation are best fits to a range of field data. Two corrections are made in the Flanagan Equation for two-phase flow:
- The value of outlet pressure is adjusted for the pressure loss due to uphill and downhill flow of two phases, including the effect of liquid holdup.
- The efficiency term is correlated to reflect measured system performance based on gas velocity and liquid-gas ratio.
For the detail of the Panhandle A equation and the Flanigan correlation, refer to chapter 10 of Gas Conditioning & Processing, Vol 1 [2]. The algorithms for computer simulation are discussed in the Gas Conditioning & Processing, Vol 3, [3].
In this TOTM (which is a continuation of the June TOTM), we will demonstrate the accuracy and application of the Flanigan correlation.
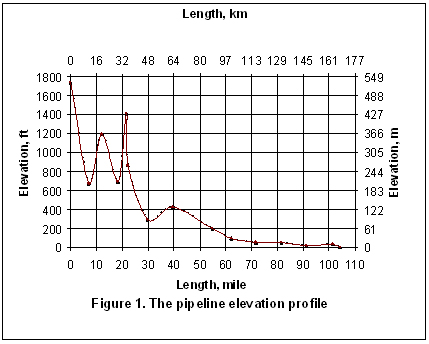
Let’s consider the same case study as was used in the June TOTM. The composition and conditions of the natural gas are shown in Table 1. The gas enters a 20 inch diameter pipeline with an inside diameter of 18.81 inches (47.8 cm) at rate of 180 MMSCFD, equivalent to 19800 lbmole/hr (8989 kgmole/h). The pipeline length and elevation profile are shown in Figure 1. The ambient temperature was assumed to be 60 °F (15.6 °C). The gas enters the line at 1165 psia (8032 kPa) and 95 °F (35 °C). The pipeline is buried under ground with an overall heat transfer coefficient of 1 Btu/hr-ft2-°F (5.68 W/m2-°C). Due to the high content of H2S and CO2 (25.6 and 9.9 mole %, respectively) and to prevent corrosion and hydrate formation, the gas has been dehydrated before entering the pipeline.
Three methods used in this analysis include the basic gas flow equation [2], the Flanigan correlation, and the computer models using the Beggs-Brill correlation with the original liquid hold-up correlation. The SRK equation of state (EOS) was used to perform the phase behavior calculations in the computer based analyses.
The pipeline is divided into 14 segments to match with the number of up-hill and down-hill sections in the line. In addition, each segment is divided into 10 equal increments to achieve higher calculation accuracy. This division is not required for the Flanigan correlation and is done for the sake of comparison with other methods.
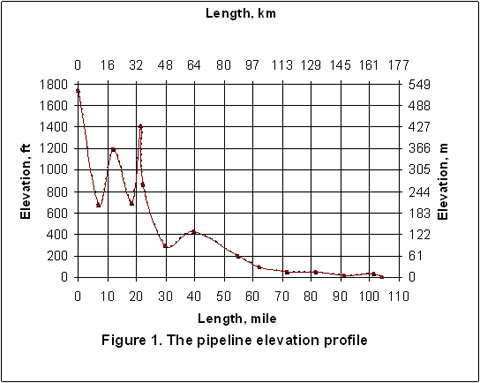
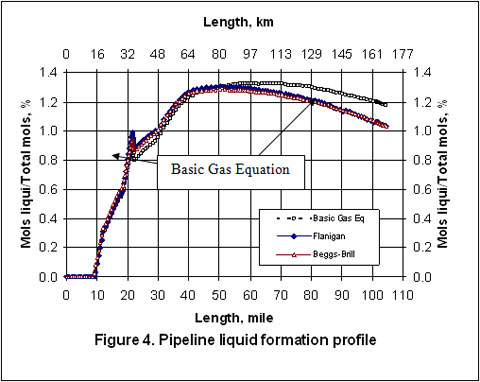
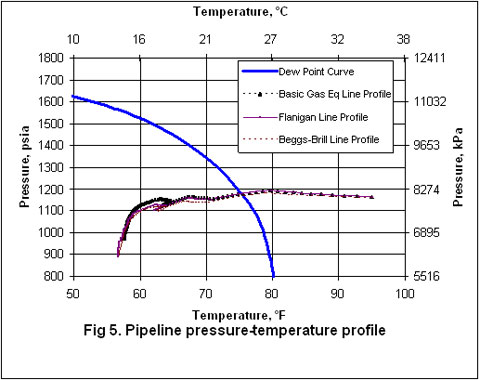
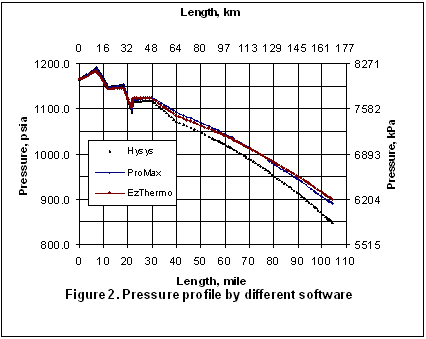
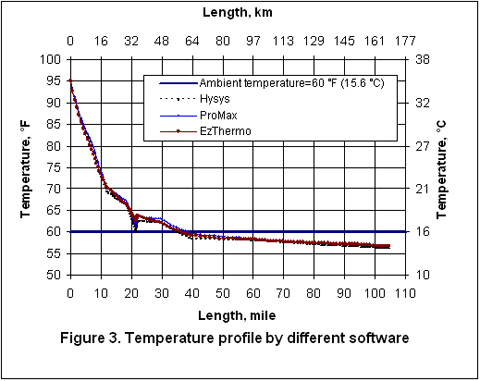
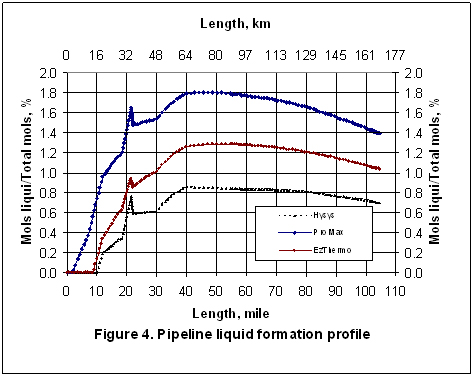
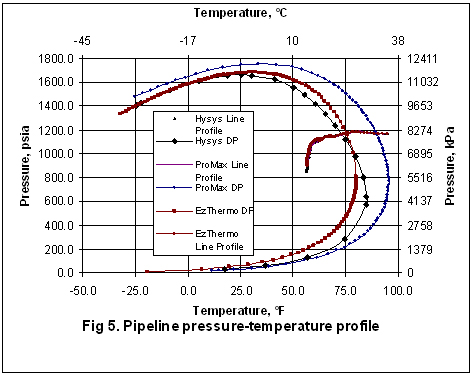
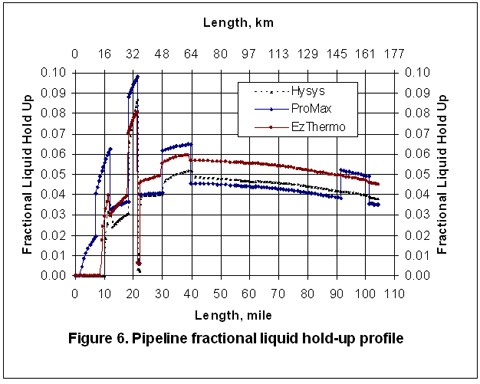
Figures 2 through 5 present the pressure, temperature, and liquid formation profiles along the pipeline. Figure 2 indicates that the pressure profiles predicted by the Flanigan matches very well with the results obtained by the more rigorous computer analyses using Beggs-Brill method. However, as expected, due to presence of liquid formation in the line, the basic gas equation results deviate from the two phase flow correlations.
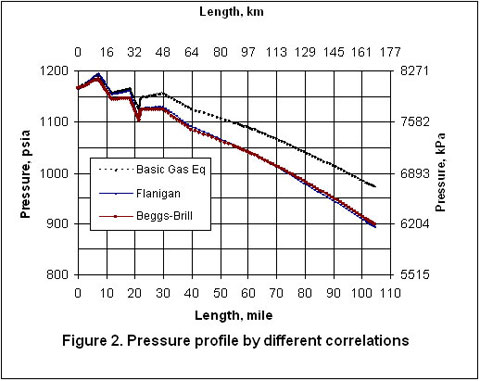
Figure 3 indicates that the temperature profiles predicted by the three correlations fall on top of each other. The small amount of liquid condensation in the line has smaller effect on the temperature profile than on the pressure profile. The liquid formation profiles predicted by the three correlations are shown in Figure 4. As shown in this figure, the amounts of liquid formation predicted by the Flanigan and Beggs-Brill correlations match very well, but the liquid formation predicted by the basic gas equation is different from the two-phase correlations. This can be explained by the fact the pressure drop and consequently the temperature change predicted by the basic equation are different from those predicted by the other two methods.
In this study, the same normal boiling point, relative density, and molecular weight for C6+, as shown in Table 1, are used for all three correlations. Therefore, the same predicted critical properties and acentric factor are used. These properties and the binary interaction parameters are needed to perform the phase behavior calculations by a cubic EOS such as SRK. In addition, the same binary interaction parameters between different components and C6+ are used.
The work reported here clearly shows the value of simple Flanigan correlation and how it can used to model and analyze the behavior of a gas transmission pipeline. However, care must be taken to utilize this correlation properly. Even though the Flanigan correlation is simple, its results match very well with the more rigorous method of Beggs-Brill. However, we expect the agreement between these two correlations deteriorate as the amount of liquid formation in the line increases. As expected the basic gas equation predicted smaller pressure drop in the line due to the fact the liquid formation in the line is ignored. Although the Flanagan Equation results are not sensitive to the elevation correction term, it is important to include the elevation term with a reasonable estimate of the total upward and downward elevation changes. The results are also relatively insensitive to the efficiency factor, therefore average values for liquid and gas ratios can be used for each segment.
Similar cases of fluid flow are discussed in our Fundamentals of Onshore and Offshore Pipeline Systems – PL-4; Onshore Pipeline Facilities – Design, Construction and Operations – PL-42; Flow Assurance for Pipeline Systems – PL-61; courses.
By: Dr. Mahmood Moshfeghian
References:
- Ellul, I. R., Saether, G. and Shippen, M. E., “The Modeling of Multiphase Systems under Steady-State and Transient Conditions – A Tutorial,” The Proceeding of Pipeline Simulation Interest Group, Paper PSIG 0403, Palm Spring, California, 2004.
- Campbell, J. M., and Hubbard, R. A., Gas Conditioning and Processing, Vol. 1 (8th Edition, 2nd Printing), Campbell Petroleum Series, Norman, Oklahoma, 2001.
- Maddox, R. N. and L. L. Lilly, Gas Conditioning and Processing, Vol. 3 (2nd Edition), Campbell Petroleum Series, Norman, Oklahoma, 1990.

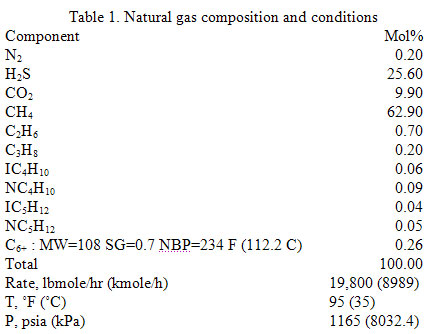


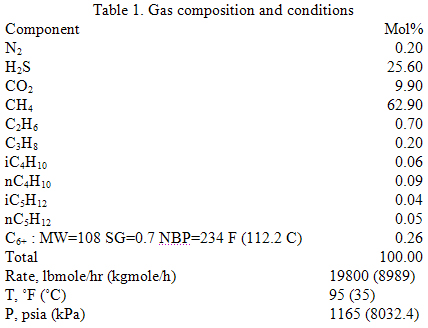
The new style of cheap authentic Seahawks jerseys in our authentic website online, not only best quality guarantee provided ,but also free shipping for all customers.
cheap Jaguars jersey