In this tip of the month (TOTM) we will describe the dense phase of a pure compound, what it is, and how it impacts processes. We will illustrate how thermophysical properties change in the dense phase as well as in the neighboring phases. The application of dense phase in the oil and gas industry will be discussed briefly. In next month TOTM, we will discuss the dense phase behavior of multi-component systems.
When a pure compound, in gaseous or liquid state, is heated and compressed above the critical temperature and pressure, it becomes a dense, highly compressible fluid that demonstrates properties of both liquid and gas. For a pure compound, above critical pressure and critical temperature, the system is oftentimes referred to as a “dense fluid” or “super critical fluid” to distinguish it from normal vapor and liquid (see Figure 1 for carbon dioxide). Dense phase is a fourth (Solid, Liquid, Gas, Dense) phase that cannot be described by the senses. The word “fluid” refers to anything that will flow and applies equally well to gas and liquid. Pure compounds in the dense phase or supercritical fluid state normally have better dissolving ability than do the same substances in the liquid state. The dense phase has a viscosity similar to that of a gas, but a density closer to that of a liquid. Because of its unique properties, dense phase has become attractive for transportation of natural gas, enhanced oil recovery, food processing and pharmaceutical processing products.
The low viscosity of dense phase, super critical carbon dioxide (compared with familiar liquid solvents), makes it attractive for enhanced oil recovery (EOR) since it can penetrate through porous media (reservoir formation). As carbon dioxide dissolves in oil, it reduces viscosity and oil-water interfacial tension, swells the oil and can provide highly efficient displacement if miscibility is achieved. Additionally, substances disperse throughout the dense phase rapidly, due to high diffusion coefficients. Carbon dioxide is of particular interest in dense-fluid technology because it is inexpensive, non-flammable, non-toxic, and odorless. Pipelines have been built to transport natural gas in the dense phase region due to its higher density, and this also provides the added benefit of no liquids formation in the pipeline.
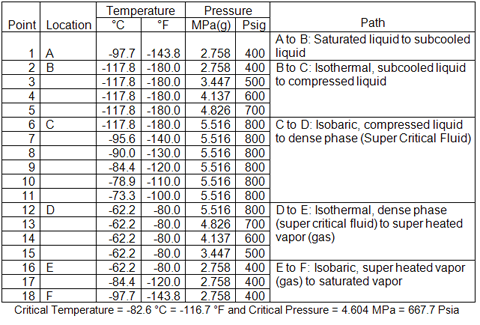
In the following section we will illustrate the variation of thermophysical properties in the dense phase and its neighboring phases. Methane properties have been calculated with HYSYS software for a series of temperatures and pressures. Table 1 presents, the pressures and temperatures and their paths used in this study.
The calculated thermophysical properties are plotted as a function of pressure and temperature in Figures 2 to 9. The thermophysical property is shown on the left-hand side y-axis, temperature on the x-axis and pressure on the right-hand side y-axis.
Table 1. Pressure-Temperature combination and the paths chosen for methane
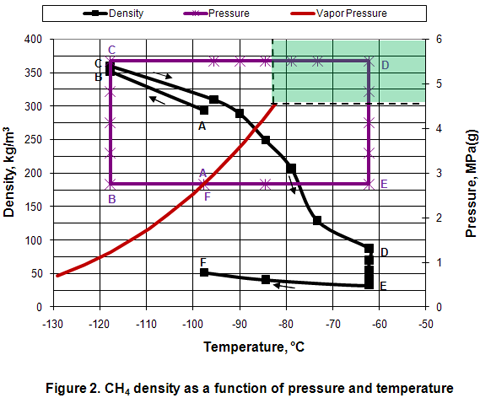
Density:
Figure 2 presents the variation of density in different phases as a function of pressure and temperature. In the isobaric subcooling path of AB, liquid density increases gradually. However, in the isothermal compression of BC path, a small increase of density is observed. In the isobaric CD path, compressed liquid density decreases gradually as temperature is increased well into the dense phase region. However, as the temperature increases further in the dense phase, density reduction is accelerated. Reduction of density is further accelerated during isothermal expansion of DE. Isobaric cooling of vapor along EF path corresponds with a gradual increase in density. It can be noted the values of dense phase density are close to the liquid phase density in some areas of the dense phase region, and is overall significantly higher than the vapor phase densities.
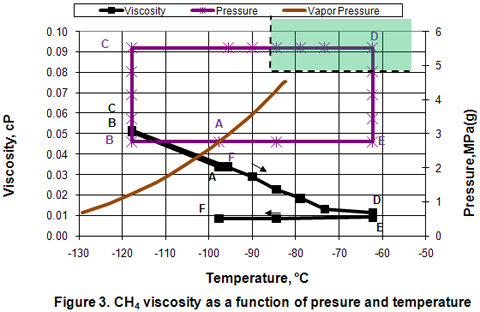
Viscosity:
Figure 3 presents the variation of viscosity in different phases as a function of pressure and temperature. In the isobaric subcooling path of AB, liquid viscosity increases rapidly. However, in the isothermal compression of BC path, a very small change of viscosity is observed. In the isobaric CD path, compressed liquid viscosity decreases linearly and sharply as temperature is increased well into the dense phase region. As the temperature increases further in the dense phase, viscosity reduction becomes gradual and approaches the gas phase values. Reduction of viscosity is quite small during isothermal expansion of DE. Isobaric cooling of vapor along EF path corresponds with no appreciable change in viscosity.
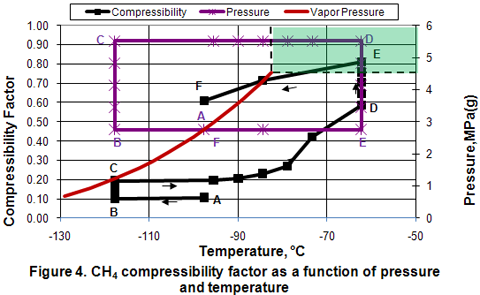
Compressibility Factor:
In general, the compressibility factor Z, calculated by an equation of state is not accurate for the liquid phase. Therefore, Figure 4 which presents compressibility factor as a function of pressure and temperature should be considered for qualitative study only. In the isobaric subcooling path of AB, Z remains almost constant. However, in the isothermal compression of BC path, Z increases drastically. In the isobaric CD path, Z increases gradually as temperature is increased well into the dense phase region. As the temperature increases further in the dense phase, the increase in Z is accelerated. The increase in Z is further accelerated during isothermal expansion of DE. Isobaric cooling of vapor along FF path corresponds with a gradual decrease in Z.
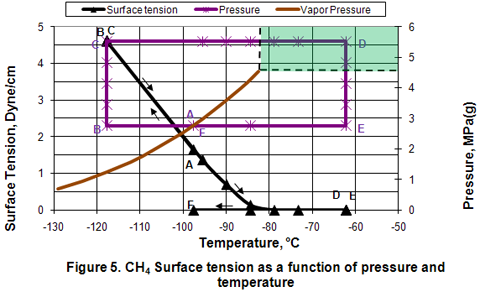
Surface Tension:
Figure 5 shows that in the liquid phase, surface tension is a strong function of temperature but independent of pressure. Above the critical temperature, surface tension is not applicable and its value is zero.
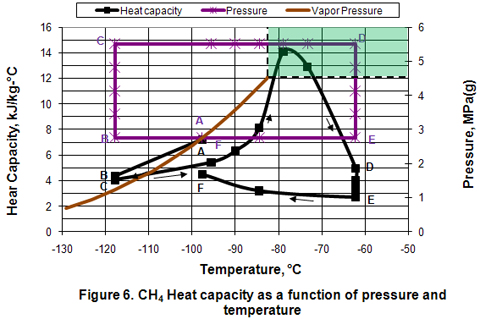
Heat Capacity:
Generally, heat capacity is applicable in a single phase region and should not be used when there is a phase change. Figure 6 presents the variation of density in different phases as a function of pressure and temperature. In the isobaric subcooling path of AB, liquid heat capacity decreases. In the isothermal compression of BC path, a small decrease of heat capacity is observed. In the isobaric CD path, compressed liquid heat capacity increases gradually as temperature is increased well into the dense phase region. As the temperature increases further in the dense phase, heat capacity reaches a maximum value and then starts to decrease. This is strange behavior and surprisingly high values are calculated. Similar results were obtained using ProMax software. Reduction of heat capacity is further noticed during isothermal expansion of DE. Isobaric cooling of vapor along EF path corresponds with a gradual increase in heat capacity.
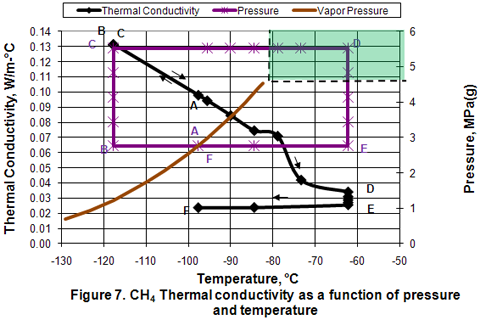
Thermal Conductivity:
Figure 7 presents the variation of thermal conductivity in different phases as a function of pressure and temperature. In the isobaric subcooling path of AB, liquid thermal conductivity increases. In the isothermal compression of BC path, no change is observed. In the isobaric CD path, compressed liquid thermal conductivity decreases gradually as temperature is increased well into the dense phase region. However, as the temperature increases further in the dense phase, thermal conductivity reduction is accelerated. Reduction of thermal conductivity is further noticed during isothermal expansion of DE. Isobaric cooling of vapor along EF path corresponds with a small decrease in thermal conductivity.
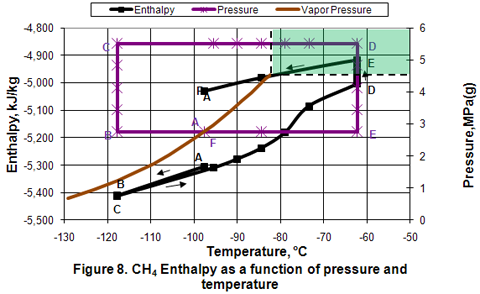
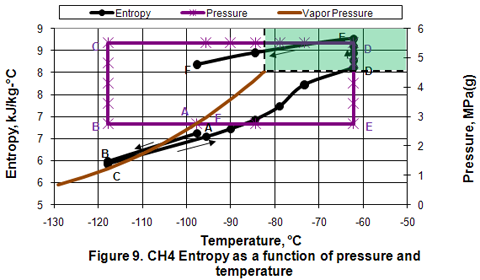
Enthalpy and Entropy:
Figures 8 and 9 present the variation of enthalpy and entropy in different phases as a function of pressure and temperature. As shown in these figures, their qualitative variations are similar. In the isobaric subcooling path of AB, liquid enthalpy and entropy decrease. In the isothermal compression of BC path, no change is observed. During the isobaric CD path, compressed liquid enthalpy and entropy values increase gradually as temperature is increased well into the dense phase region. However, as the temperature increases further in the dense phase, the enthalpy and entropy increase becomes larger. The increase in enthalpy and entropy is further noticed during isothermal expansion of DE. Isobaric cooling of vapor along EF path corresponds with a decrease in enthalpy and entropy.
Conclusions:
Dense phase behavior is unique and has special features. The thermophysical properties in this phase may vary abnormally. Care should be taken when equations of state are used to predict thermophysical properties in dense phase. Evaluation of equations of state should be performed in advance to assure their accuracy in this region. Many simulators offer the option to use liquid-based algorithms (e.g. COSTALD) for this region.
As shown in Figure 1, there is a gradual change of phase transition from gas-to-dense and dense-to-liquid phases or vice versa. Dense phase is a highly compressible fluid that demonstrates properties of both liquid and gas. The dense phase has a viscosity similar to that of a gas, but a density closer to that of a liquid. This is a favorable condition for transporting natural gas in dense phase as well as carbon dioxide injection into crude oil reservoir for enhanced oil recovery.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), PF81 (CO2 Surface Facilities), and PF4 (Oil Production and Processing Facilities) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Mark Bothamley and Mahmood Moshfeghian
Reference:
- ASPENone, Engineering Suite, HYSYS Version 2006, Aspen Technology, Inc., Cambridge, Massachusetts U.S.A., 2006.

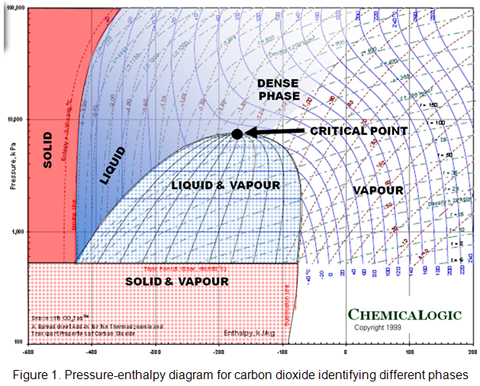
[…] fluid” to distinguish it from normal vapor and liquid (see Figure 1 for carbon dioxide in December 2009 TOTM [1]). Dense phase is a fourth (Solid, Liquid, Gas, Dense) phase that cannot be described by the […]
[…] M., “Variation of properties in the dense phase region; Part 1 – Pure compounds,” http://www.jmcampbell.com/tip-of-the-month/2009/12/variation-of-properties-in-the-dense-phase-region…, December […]
[…] fluid” to distinguish it from normal vapor and liquid (see Figure 1 for carbon dioxide in December 2009 TOTM […]
[…] Para un compuesto puro, por encima de la presión y temperatura crítica, se le refiere al sistema con frecuencia como uno de “fase densa”, o fluido super – crítico para diferenciarlo de un vapor o líquido normal (véase la Figura 1 para el dióxido de carbono en el PDM de Diciembre 2009 [1]). […]
[…] fluid” to distinguish it from normal vapor and liquid (see Figure 1 for carbon dioxide in December 2009 TOTM […]
[…] M., “Variation of properties in the dese phase region; Part 1 – Pure compounds,” TOTM, http://www.jmcampbell.com/tip-of-the-month/2009/12/variation-of-properties-in-the-dense-phase-region…, Dec […]
Submit Comment
[…] or “super critical fluid” to distinguish it from normal vapor and liquid (see Figure 1 in December 2009 Tip Of The Mont (TOTM) [1] for carbon dioxide and in January 2010 TOTM [2] for a typical natural gas). Dense phase is a […]
[…] fluid” to distinguish it from normal vapor and liquid (see Figure 1 for carbon dioxide in December 2009 TOTM […]
[…] M., “Variation of properties in the dese phase region; Part 1 – Pure compounds,” TOTM, http://www.jmcampbell.com/tip-of-the-month/2009/12/variation-of-properties-in-the-dense-phase-region…, Dec […]
Buenos días me he leído tu post y me apetecía agradecerte que hayas gastado tu tiempo en recopilar toda esta información tan necesaria para los que nos encontramos perdiendo peso. gracias por tu articulo!! Y un saludo!!
Good post. I am a regular visitor of your web site and appreciate you taking the time to maintain the nice site. I will be a regular visitor for a long time.
In first & 2nd diagrams AB & CD are referred as isobaric processes although pressure seems to change from approx 4.4 MPa(g) to 5.4 MPa(g) in case of AB (as indicated by black line).
Letto, ottime informazioni buona giornata
Loving the information on this web site, you have done outstanding job on the blog posts.
I dugg some of you post as I thought they were invaluable extremely helpful