In the February 2010 tip of the month (TOTM) we presented the distribution and concentration of sulfur-containing compounds in an NGL Fractionation (NF) plant using HYSYS [1] with the Peng-Robinson equation of state (PR EOS) [2]. In this TOTM we will present the distribution and concentration of the sulfur-containing compounds in the same NF plant using ProMax [3] and VMGSim [4] both using the PR EoS. These two simulation results will be compared with the HYSYS [1] results. The software’s built-in binary interaction parameters were used in this study. The NF plant is the same as the one described by Alsayegh et al. [5]. The feed composition, rate, condition, and product specifications are shown in Tables 1 and 2 and the plant process flow diagram is shown in Figure 1 of the February 2010 TOTM. An overall tray efficiency of 90 percent was used for all columns.
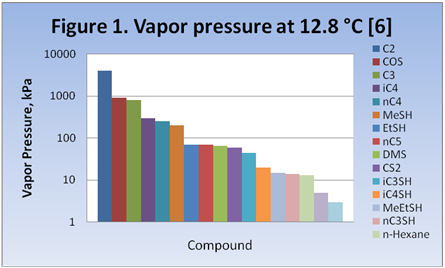
Expected Product Distribution: Figure 1, reproduced from Figure 9 of a paper published by Likins and Hix [6], shows a descending order log scale bar-graph of the pure compounds vapor pressure for the components of interest to this study. This figure shows that COS should distribute to both the ethane and the propane streams. MeSH, with a vapor pressure close to n-butane should distribute primarily with the butanes with a small amount distributing to the pentane stream. EtSH, having a vapor pressure between butane and pentane, should distribute primarily with butane and pentane. CS2 should distribute primarily to the pentane and the C6+ streams with only minor distribution to the butane stream. The heavier sulfur compounds should end up almost entirely in the C6+ stream.
Results of Computer Simulation:
The NF plant described in the previous section was simulated using HYSYS [1], ProMax and VMGSim based on the PR EOS [2]. In this study, the respective software built-in (library) binary interaction parameters were used even though we recommend evaluating the accuracy of VLE results against experimental data and if necessary the insertion of VLE data regression into the EOS interaction parameters. This regression may be required to adequately model the systems dealing with mercaptans.
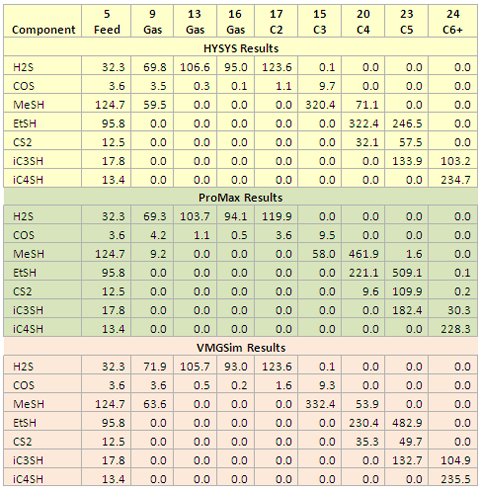
- Table 1. Concentration (PPM, mole) of sulfur containing compounds in the gas and product streams
The focus of this study is on the distribution (% recovery) and concentration (PPM) of the sulfur-containing compounds in the product streams. Table 1 presents the PPM concentration of sulfur-containing compounds in the feed and product streams. Figures 2 through 8 present bar-graphs of the recovery of each sulfur-containing compound in the gas and product streams. The mole percent recovery is defined as the number of moles of a component in the product stream divided by the moles of the same component in the feed stream (Stream 5). In these figures, the gas and product streams are followed by letters H, P, and V representing HYSYS, ProMax, and VMGSim results, respectively.
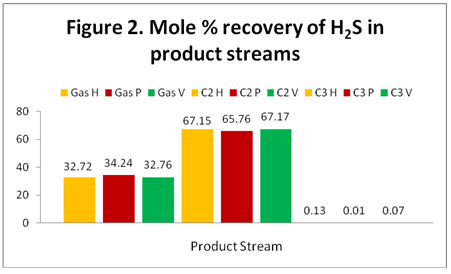
H2S: Figure 2 shows the distribution and recovery of H2S in the gas, C2 and C3 product streams. As expected, the majority of the H2S distributes in the gas and the C2 product streams. As can be seen in this figure, the results of the simulators are the same.
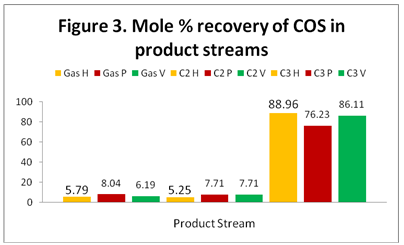
COS: Figure 3 shows the distribution and recovery of COS in the gas, C2, and C3. As expected, the majority of the COS ends up in the C3 product stream. As can be seen in this figure, the results of the three simulators are almost the same.
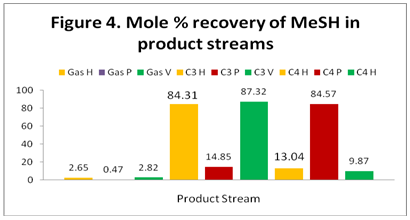
MeSH: Figure 4 shows the distribution and recovery of MeSH in the gas, C3, and C4 product streams. For HYSYS and VMGSim, contrary to the data presented in Figure 1, the majority of the MeSH distributes to the C3 stream rather than to the C4 stream. However, the ProMax result follows the same trend as in Figure 1 and the majority of MeSH distributes to the C4 stream.
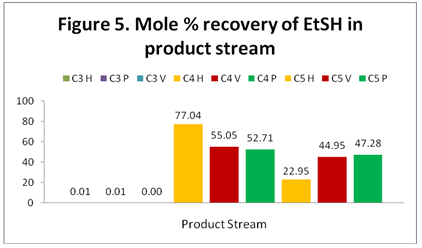
EtSH: Figure 5 shows the distribution and recovery of EtSH in the C3, C4, and C5 streams. Unexpectedly, HYSYS predicts that the majority of the EtSH ends up in the C4 stream rather than the C5 product as would be expected based on the data of Figure 1. However, the results of ProMax and VMGSim are closer to the Figure 1 data.
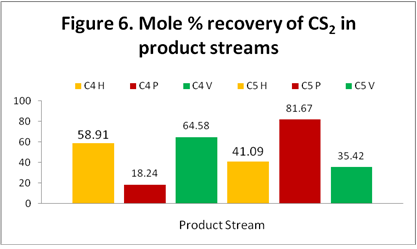
CS2: Figure 6 shows the distribution and recovery of CS2 in the C4 and C5 product streams. Contrary to the Figure 1 pure CS2 behavior the results of HYSYS and VMGSim show that the majority of the CS2 ends up in the C4 stream. However, based on the ProMax results, the majority of the CS2 ends up in the C5 stream which is consistent with data in Figure 1.
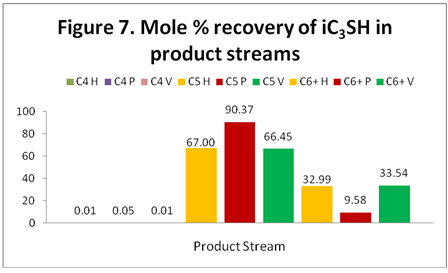
iC3SH: Figure 7 shows the distribution and recovery of iC3SH in the C4, C5 and C6+ product streams. As expected, iC3SH ends up in the C5 and C6+ streams. Notice that ProMax shows a higher concentration of iC3SH in the C5 product stream while HYSYS and VMGSim predict lower but nearly the same recovery of iC3SH.
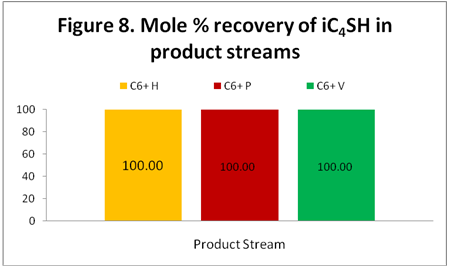
iC4SH: Figure 8 shows recovery of iC4SH in the C6+ product stream. All of the iC4SH ends up in the C6+ stream as expected when the Figure 1 data is analyzed.
Conclusions:
The calculation results presented and discussed here are specific to the NGL fractionation plant studied here, but there are some general conclusions that can be drawn from this study.
The results indicate that the highest concentration of methyl mercaptan (MeSH) is present in the C3 product (stream 15) based on HYSYS and VMGSim but its highest concentration is in the C4 product (stream 20) based on the ProMax results.
The results of HYSYS indicate that the highest concentration of ethyl mercaptan (EtSH) is present in the C4 product (stream 20) but ProMax and VMGSim results indicate that its highest concentration occurs in the C5 Product (stream 23).
The highest concentration of carbon disulfide (CS2) is present in C5 Product (stream 23) according to the three simulator results.
The binary interaction parameters used in the EOS play an important role in the VLE behavior of the system under study, and affect the distribution of the sulfur-containing compounds present in the feed. Use of improper or incorrect binary interaction parameters may generate erroneous results. Care must be taken to use correct values of binary interaction parameters. In this study, the simulator library values of the binary interaction parameters were used.
The predictions by HYSYS, ProMax, and VMGSim in Figures 4 through 7 (showing the distribution of MeSH, EtSH, CS2, and iCH3SH respectively) contain some disagreements. The results also indicate that these compounds were not distributed among the hydrocarbon products in the same way one would expect from their volatilities and concentrations. This may be explained by the conclusion reported by Harryman and Smith [7, 8] who wrote “iC3SH is formed during fractionation within the depropanizer and the deethanizer.” Therefore, further evaluation should be conducted to arrive at a concrete decision. In an upcoming TOTM, we will investigate the VLE behavior of the theses systems using experimental data. This should be a good reason to perform laboratory tests and detailed thermodynamic calculations to determine process flow rates and composition. Detailed process analysis shouldalways be made to justify and prove correct decisions as to selection of process flow schemes.
To learn more about similar cases and how to minimize operational problems, we suggest attending the John M. Campbell courses; G4 (Gas Conditioning and Processing), G5 (Gas Conditioning and Processing – Special) and G-6 Gas Treating and Sulfur Recovery.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- ASPENone, Engineering Suite, HYSYS Version 7.0, Aspen Technology, Inc., Cambridge, Massachusetts U.S.A., 2009.
- Peng, D.,Y. and D. B. Robinson, Ind. Eng. Chem. Fundam. 15, 59-64, 1976.
- ProMax 3.1, Bryan Research and Engineering, Inc, Bryan, Texas, 2009.
- VMGSim 5.0.5, Virtual materials Group, Inc, Calgary, Alberta, 2010.
- Al-Sayegh, A.R., Moshfeghian, M. Abbszadeh, M.R., Johannes, A. H. and R. N. Maddox, “Computer simulation accurately determines volatile sulfur compounds,” Oil and Gas J., Oct 21, 2002.
- Likins, W. and M. Hix, “Sulfur Distribution Prediction with Commercial Simulators,” the 46th Annual Laurance Reid Gas Conditioning Conference Norman, OK 3 – 6 March, 1996.
- Harryman, J.M. and B. Smith, “Sulfur Compounds Distribution in NGL’s; Plant Test Data – GPA Section A Committee, Plant design,“ Proceedings 73rd GPA Annual Convention, New Orleans, Louisiana, March, 1994.
- Harryman, J.M. and B. Smith, “Update on Sulfur Compounds Distribution in NGL’s; Plant Test Data – GPA Section A Committee, Plant design,“ Proceedings 75th GPA Annual Convention, Denver, Colorado, March, 1996.

Dr. Mahmood, this is a very interesting and practical post. I notice that, in practice, MeSH would distribute to propane and butane, even though based on vapor pressure alone it should go to butane and heavier fractions. This is because of the non-ideality behavior of MeSH.
Maria;
There is no question that the non-ideal behavior influences the distribution of mercaptans. However, simple rules such as Raoult’s law and boiling point distribution provide useful guide and point us to the right direction. Hopefully with current research underway and collection of more experimental data we find more accurate and firm conclusion.
Dear Dr Mahmood, I am from VMG Europe and I have reproduced the simulation in this post. It is found that by using VMGSim we are able to capture the non-ideal behavior of MeSH, along with the ideal behavior of other sulfur containing compounds.
This result is backed up by several qualitative scientific paper. Hopefully the future research will be able to give a more quantitative experimental data. Thank you for the post and the reply, hope everything goes well with you.