For dehydration of natural gas by triethylene glycol (TEG) process to a lower water content/water dew point temperature a higher lean TEG concentration is required. To achieve a higher lean TEG concentration at a specified reboiler temperature and pressure, commonly a stripping gas is used. Stripping is defined as a physical separation process by which one or more components are removed from a liquid stream by a vapor stream. Stripping gas lowers the water partial pressure causing more water to vaporize from TEG solution. Normally, stripping is performed at the practical/possible highest temperature and lowest pressure. This allows for minimum stripping gas flow rate. Any inert gas or a portion of the gas being dehydrated is suitable.
In this Tip Of The Month (TOTM), the impact of stripping gas on the water partial pressure and lean TEG solution boiling temperature or lean TEG concentration is studied. Two case studies are considered. In case one, at a specified reboiler temperature and pressure, the impact of stripping gas on lowering the water partial pressure and consequently increasing the TEG concentration in the lean TEG solution is demonstrated. Similarly in case two, for a specified 99 and 99.4 mass percent TEG in the liquid phase and atmospheric pressure, the impact of stripping gas on lowering the water partial pressure and consequently lowering the liquid boiling point (bubble point) temperature is demonstrated.
Glycol dehydration is the most common dehydration process used to meet pipeline sales specifications and field requirements (gas lift, fuel, etc.). TEG is the most common glycol used in these absorption systems. At atmospheric pressure and a maximum reboiler temperature of 204 °C [400 °F] the highest glycol concentration of lean TEG that can be achieved is roughly 99 mass percent. This represents the maximum lean TEG concentration that can be produced in a reboiler operating at 1 atm. If the lean TEG concentration required at the absorber to meet the water dew point specification is higher than 99 mass percent, then some method of further increasing the glycol concentration at the regenerator must be incorporated in the unit. Virtually all of these methods involve lowering the partial pressure of water in the glycol solution either by pulling a vacuum on the regenerator or by introducing stripping gas into the regenerator [1].
In the June and July 2013 Tip Of The Months (TOTM) [2, 3], the effect of stripping gas rate on the regenerated lean TEG concentration for several operation conditions was studied. A series of charts and their corresponding correlations for quick determination of the required stripping gas rate to achieve a desired level of lean TEG concentration were presented. The charts were based on the rigorous calculations performed by computer simulations with correlations developed by regressing the same data to a proposed model. These can be used for facilities type calculations for evaluation and trouble shooting of an operating TEG dehydration unit. For further detail refer to the June and July 2013 TOTM [2, 3].
Vapor-Liquid Equilibrium:
For the sake of simplicity, pure methane is used as the stripping gas; however, the following discussion is valid for any gas mixture.
The governing equations for vapor-liquid equilibrium of TEG, water, and methane are derived and presented in Appendix A at the end of this TOTM. Even though the system of water, TEG and methane is non-ideal, to show the principle of gas stripping, assume the vapor phase is an ideal gas and the liquid phase is an ideal solution. Under these conditions Raoult’s Law for water (See Eq A5 in Appendix A) is.
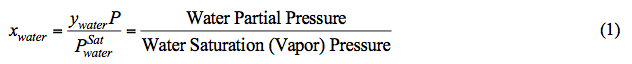
According to Raoult’s Law, at a fixed pressure and temperature (i.e. the reboiler condition), the mole fraction of water in the liquid phase (xwater) decreases as water partial pressure decreases. Note that at a fixed temperature, water vapor pressure is constant (see Figure 1). In addition, system pressure (P) is the sum of partial pressure of all components. Assuming an ideal mixture, mathematically P is approximated by:
Figure 1 presents the vapor pressure of water [4] and TEG [5] for an operating range of reboiler temperature. As can be seen in Figure 1, the vapor pressure (volatility) of water is much higher than that of TEG. In general the vapor pressure of water is approximately 100 to 1000 times greater than that of TEG. This shows why water is vaporized much more than TEG from a TEG solution by heating.
Since TEG volatility is much lower than that of water and methane, its mole fraction in the vapor phase will be much smaller and for simplicity its partial pressure can be ignored. Therefore equation 2 reduces to:
![]()
At a fixed system pressure (reboiler condition), equation 3 clearly shows that as the methane partial pressure increases, water partial pressure has to decrease and consequently, based on Raoult’s Law, equation 1, the mole fraction of water in the liquid phase decreases. In other words, increasing the stripping gas rate results in lower water mole fraction in TEG and higher TEG mole (mass) fraction is achieved.
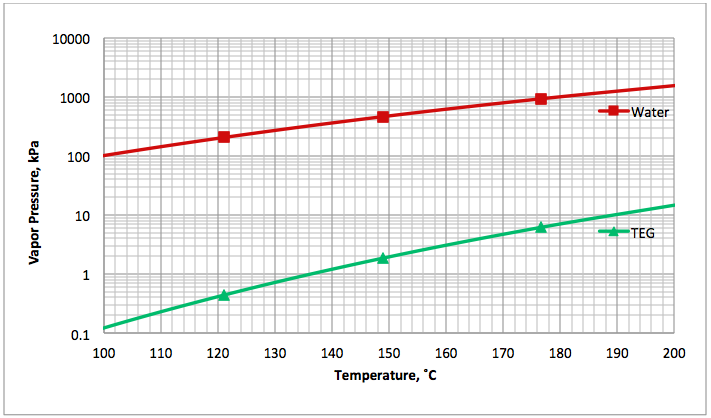
Fig 1A (SI). Comparison of water [4] and TEG [5] vapor pressure
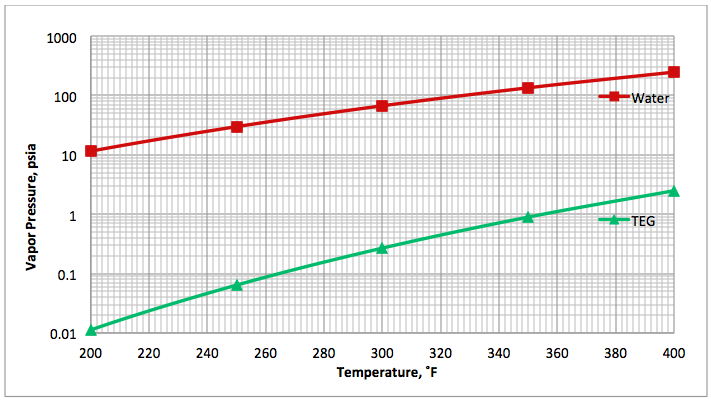
Fig 1B (FPS). Comparison of water [4] and TEG [5] vapor pressure
The effect of stripping gas can be summarized as follows:
- Higher stripping gas (methane) rate increases methane mole fraction in the vapor phase
- Higher methane mole fraction in the vapor phase increases partial pressure of methane
- Higher partial pressure of methane lowers partial pressure of water (P is fixed, Eq 3)
- Lower partial pressure of water lowers mole fraction of water (Raoult’s Law, Eq 1) in the liquid phase (Raoult’s Law, Eq 1) which, in turn, increases the TEG concentration
In order to demonstrate quantitatively the impact of stripping gas on water partial pressure and lean TEG concentration or boiling temperature and to support the above reasoning, two case studies were investigated. For both cases, ProMax [6] with the SRK (Soave-Redlich-Kwong) [7] equation of state option was used to perform the calculations. In addition, all of the results presented are are for a single equilibrium (theoretical) stage.
Case Study 1:
In this case, the impact of stripping gas on regeneration of lean TEG concentration at reboiler conditions of 101.3 kPa and 199°C (14.7 psia & 390°F) is studied.
Figures 2 A & B present the variation of partial pressures of TEG, water and methane as a function of stripping gas (methane) rate and concentration, respectively. The total pressure is constant and plotted in this figure as well. These figures clearly show that as stripping gas (methane) rate or concentration increases, the methane partial pressure increases and the water partial pressure decreases, approximately in the same amount. Note the TEG partial pressure is relatively constant and very low compared to methane and water. It makes up less than 7 percent of the total pressure.
Figures 3 A & B present the impact of stripping gas rate and concentration in the vapor and liquid phases on lean TEG concentrations. As can be seen in these figures, as the stripping gas rate (or concentration) increases, the lean TEG concentration increases. It should be emphasized that these results are for a single equilibrium stage. As shown in the previous TOTM, increasing the number of stripping stages changes this relationship significantly.
Figures 2 and 3 indicate that variation of methane and water partial pressures or lean TEG concentration with stripping gas (methane) rate is non-linear whereas their variation with stripping gas concentration is linear.
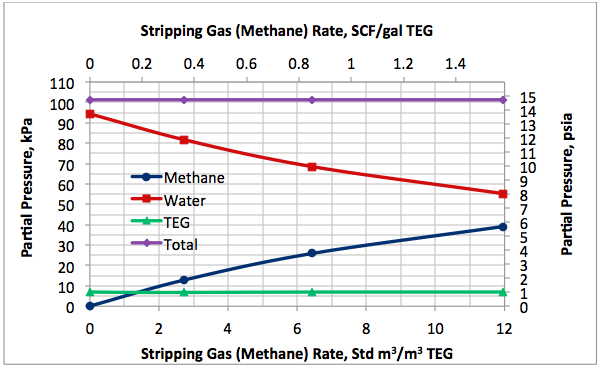
Fig 2A. Impact of stripping gas rate on partial pressure of methane, water and TEG at 101.3 kPa & 199°C (14.7 psia & 390°F)
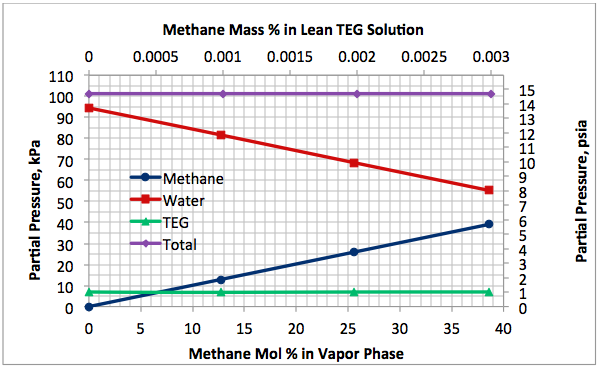
Fig 2B. Impact of stripping gas concentration on partial pressure of methane, water and TEG at 101.3 kPa & 199°C (14.7 psia & 390°F)
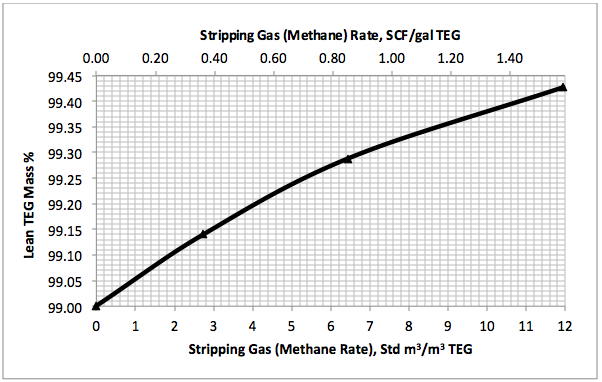
Fig 3A. Impact of stripping gas rate on lean TEG concentration at 101.3 kPa & 199°C (14.7 psia & 390°F)
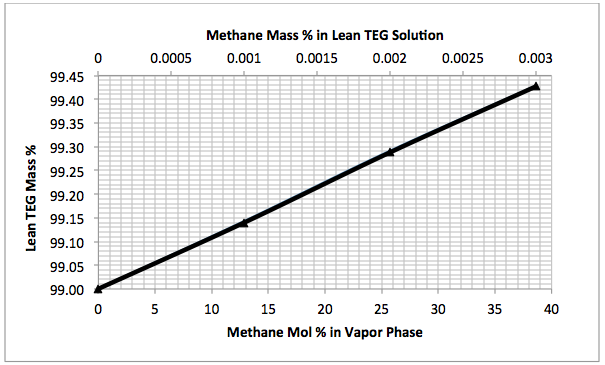
Fig 3B. Impact of stripping gas concentration on lean TEG concentration at 101.3 kPa & 199°C (14.7 psia & 390°F)
Case Study 2:
This is an academic case and is presented to reemphasize the impact of stripping gas. In this case, the impact of stripping gas concentration on the lean TEG solution boiling (bubble point) temperature for relatively fixed lean TEG concentration of TEG and water at 101.3 kPa (14.7 psia) is studied.
Figure 4 indicates that as the stripping gas (methane) concentration increases, the lean TEG solution boiling (bubble point) temperature drops.
Figure 5 presents a variation of partial pressures of TEG, water and methane as a function of the stripping gas (methane) concentration. The total pressure is constant and plotted in this figure, too. As in case 1, this figure also clearly indicates that as the stripping gas (methane) concentration increases, while methane partial pressure increases water partial pressure decreases, approximately in the same amount. Note that TEG partial pressure decreases due to the lowering of boiling temperature and its value is much lower compared to those of methane and water. Again, it makes up less than 7 percent of total pressure.
Variation of component K-values as a function of TEG solution boiling (bubble point) temperature is presented in Figure 6. This figure indicates that the relative volatility of water with respect to TEG (Kwater/KTEG) is in the order of 100 and that of methane with respect to TEG (Kmethane/KTEG) is in the order of more than 10,000.
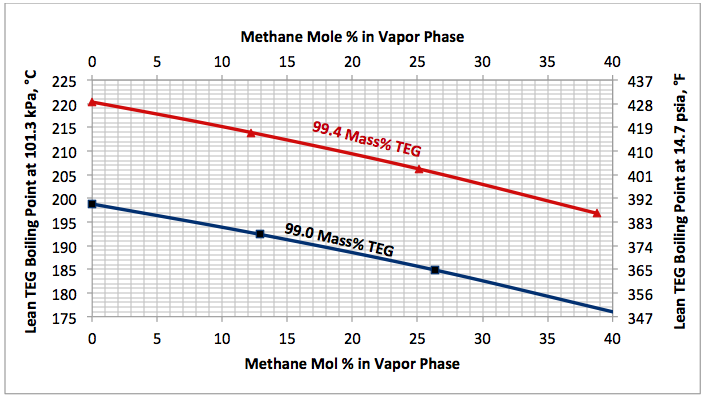
Fig 4. Impact of stripping gas concentration on the lean TEG solution boiling (bubble point) temperature at 101.3 kPa (14.7 psia).
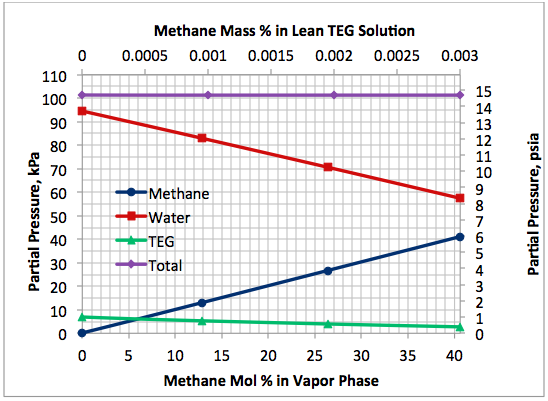
Fig 5. Impact of stripping gas concentration on the component partial pressures at 101.3 kPa (14.7 psia) for 99 mass % TEG and 1 mass % water.
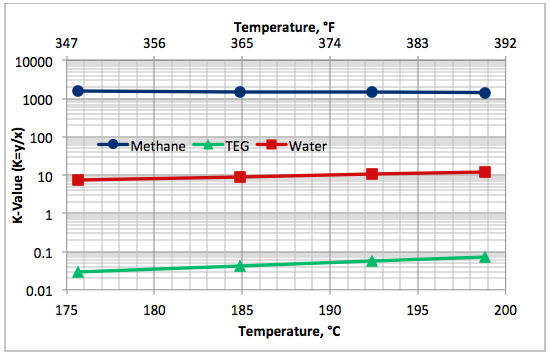
Fig 6. Variation of K-values with the lean TEG solution boiling (bubble point) temperature at 101.3 kPa (14.7 psia) for 99 mass % TEG and 1 mass % water.
Example 1:
Determine the reboiler temperature to regenerate a lean TEG concentration of 99.4 mass % at 101.3 kPa (14.7 psia). If the required reboiler temperature is above the maximum allowable of 204°C (400°F), determine the required partial pressure of a stripping gas (methane) to keep the reboiler temperature at 199°C (390°F).
Solution:
Figure 4 indicates that for a lean TEG concentration of 99.4 mass %, the required reboiler temperature without any stripping gas is 220.5°C (428.8°F). Obiviously, the is above the maximum allowable operating reboiler temperature. From the same figure, for a reboiler temperature of 199°C (390°F), the required mole % of striping gas in the vapor phase is 35.6. Therefore, the required partial of stripping gas is:
![]()
Example 2:
It is desired to regenerate a lean TEG solution to a concentration of 99.4 mass % at 101.3 kPa and 199°C (14.7 psia & 390°F). How much stripping gas is required in a regenerator with 1 theoretical tray?
Soultion:
Figure 3A indicates that for a lean TEG concentration of 99.4 mass%, the required striping gas rate is 10.9 Std m3/m3 of TEG (1.44 SCF/gal TEG). The coressponding water and methane partial pressures from Figure 2A are 57.8 and 36.5 kPa ( 8.4 and 5.3 psia), respectively. Note that without any stripping gas, the maximum achievable lean TEG concentration is 99 mass % and the coressponding water and methane partial pressures are 94.6 and 0 kPa (13.7 and 0 psia), respectively. The stripping gas reduced the water partial presuure by 38.9 %.
Conclusions:
To achieve a higher lean TEG concentration at a specified reboiler temperature, i.e. below maximum of 204°C (400°F), and pressure, a stripping gas is commonly used. In this TOTM, the mechanism of stripping gas was reviewed. The impact of stripping gas on lean TEG solution concentration or boiling (bubble point) temperature was further investigated quantitatively. The impact of stripping gas on regeneration of TEG solution concentration can be summarized as:
- Each constituent of a mixture exerts its own partial pressure as a function of temperature and composition.
- Total system pressure is the sum of all partial pressures.
- For a fixed pressure, stripping gas lowers the partial pressure of water in the vapor phase
- Even though this system is not ideal, from Raoult’s Law (equation 1) at a fixed pressure and temperature the concentration of water in the TEG solution decreases as the partial pressure in the vapor phase decreases
To learn more, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), G5 (Gas Conditioning and Processing-Special), and PF81 (CO2 Surface Facilities), PF4 (Oil Production and Processing Facilities), courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.com, or email us at consulting@jmcampbell.com.
By: Dr. Mahmood Moshfeghian
Reference:
- Campbell, J. M., “Gas Conditioning and Processing”, Vol. 2, The Equipment Module, 8th Ed., Second Printing, J. M. Campbell and Company, Norman, Oklahoma, 2002.
- Moshfeghian, M., http://www.jmcampbell.com/tip-of-the-month/2013/05/teg-dehydration-stripping-gas-charts-for-lean-teg-regeneration/, June 2013.
- Moshfeghian, M., http://www.jmcampbell.com/tip-of-the-month/2013/06/teg-dehydration-stripping-gas-correlations-for-lean-teg-regeneration/, July 2013.
- Smith, J.M., Van Ness, H.C. and M.M. Abbott, “Introduction to Chemical Engineering Thermodynamics,” 7th, McGraw-Hill, New York, 2004.
- http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_004d/0901b8038004d042.pdf, Dow Chemical Company, 2007.
- ProMax 3.2, Bryan Research and Engineering, Inc., Bryan, Texas, 2013.
- Soave, G., Chem. Eng. Sci. Vol. 27, No. 6, p. 1197, 1972.
Appendix A: Vapor-Liquid Equilibrium
The criteria for vapor-liquid equilibrium are the equality of fugacity of each component in the mixture between phases. Applying these criteria to fugacity (f), of component (i) in the vapor (V) and liquid, (L), phases:
![]()
For a non-ideal mixture of water, TEG and methane, one can write the following expressions in terms of fugacity coefficients (φ) in the vapor phase and activity coefficients (γ) in the liquid phases.
![]()
And,
![]()
Where:

Equating equations A2 and A3 and solving for xi:
![]()
Calculation of ![]() involves the use of an equation of state. It is a tedious trial and error procedure; therefore, a computer program should be used. These two terms represent the non-ideality of the vapor phase and if the system is an ideal gas both are set equal to 1. On the other hand, activity coefficient represents the non-ideality of the liquid phase and is calculated by an appropriate activity coefficient model. For an ideal liquid solution, the activity coefficient is also set to 1.
involves the use of an equation of state. It is a tedious trial and error procedure; therefore, a computer program should be used. These two terms represent the non-ideality of the vapor phase and if the system is an ideal gas both are set equal to 1. On the other hand, activity coefficient represents the non-ideality of the liquid phase and is calculated by an appropriate activity coefficient model. For an ideal liquid solution, the activity coefficient is also set to 1.
Assuming the vapor phase is an ideal gas and the liquid phase is an ideal solution, then equation A4 reduces to equation A5, which is Raoult’s Law.
![]()

Very interesting article on the effect of stripping gas on the TEG purity. This is often one of the most misunderstood concept in dehydration and lot of stripping gas is used without any purpose.
There has been a few articles on TEG dehydration all of which are really informative.
One area I would like to see addressed is the issue of TEG condensation in transmission pipelines. There are very few VLE data to support a good analysis and the industry is quite divided on how much TEG which is in equilibrium at the contactor outlet (and assuming any entrained TEG is removed in the downstream filter coalescer) condenses in the pipeline. Appreciate if some information can be shared on this.
Once again a big thank you to Dr. Mahmood Moshfeghian
for sharing his espertise.
Thank for revealing this useful information about TEG techniques.I appreciate
can N2 be used instead of natural gas for stripping media in glycol regeneration system?
can N2 used instead of natural gas for stripping in glycol regeneration system.
[…] M., http://www.jmcampbell.com/tip-of-the-month/2013/08/teg-dehydration-how-does-the-stripping-gas-work-i…, Tip of the Month, August […]
Dear,
Maybe an off-topic question, but why is Methane used as a stripping agent and not a different compound like CO2?
alot of information on the use of methane but nothing about an other compound.
Thanks in advance
It’s just a test.
May I simply just say what a relief to uncover somebody that truly knows what they are talking about on the web.
You definitely know how to bring an issue to light and make it important.
More and more people must check this out and understand this
side of your story. I was surprised you are not more popular since
you definitely have the gift.
I think the admin of this site is actually working hard for his web site,
since here every data is quality based information.
Can I simply say what a comfort to find an individual who genuinely understands what they are discussing on the internet.
You certainly realize how to bring an issue to light and make it important.
A lot more people should look at this and understand this side of the story.
I can’t believe you are not more popular given that
you most certainly possess the gift.
Hi Mate, I am commenting from Orange Australia. We have had a lot of flooding the last few months and I’ve only just been able to connect to the blogsphere Thanks so much for the easy to read blog. It inspired me a lot with my college cooking essay. God Bless the internet !
Hello I am so glad I found your blog, I really found you by error, while I was searching on Digg for something else, Anyways I am here now and would just like to say thanks a lot for a tremendous post and a all round thrilling blog (I also love the theme/design), I don’t have time to read through it all at the moment but I have saved it and also added in your RSS feeds, so when I have time I will be back to read a great deal more, Please do keep up the superb job.
Timely piece – I Appreciate the info . Does someone know if I would be able to access a blank 2011 US NAVPERS 1336/3 example to fill out ?
Intresting.Will want more of this journal because it relates to my work
keep up the excellent piece of work, I read few blog posts on this site and I conceive that your web site is real interesting and holds bands of excellent information.
This is a interesting blog and I really enjoy it very much. For your readers I have an extremely useful Facebook fanpage. Thanks.
I am really enjoying the theme/design of your blog. Do you ever run into any web browser compatibility problems? A number of my blog visitors have complained about my blog not working correctly in Explorer but looks great in Opera. Do you have any tips to help fix this issue?
DreamProxies.com : cheapest high level private proxies with 50% price cut! Professional quality, Limitless proxies, Extremely speed and also Least expensive prices – only $0.25 per proxy! Best non-public proxies simply from DreamProxies.com
DreamProxies.com – most affordable high level private proxies with 50% low cost! Professional quality, Limitless proxies, Excellent speed and also Cheapest prices — simply $0.25 each proxy! Finest exclusive proxies just by DreamProxies.com
Thanks, I’ve recently been hunting for facts about this topic for ages and yours is the best I have discovered so far.
Please why do the stripping gas come from the bottom
The core of your writing while sounding agreeable in the beginning, did not really work perfectly with me personally after some time. Someplace throughout the paragraphs you actually managed to make me a believer but just for a while. I however have a problem with your jumps in assumptions and one might do nicely to help fill in all those gaps. In the event that you can accomplish that, I will undoubtedly end up being amazed.
Thanks. Actually, I didn’t figure out exact reason why and how this stripping gas is required at GDU. Your articl is really helpful to me.
If it is possible to replace stripping gas agent from fuel gas to nitrogen
If it is possible to replace stripping gas agent from fuel gas to nitrogen
Hurrah, that’s what I was exploring for, what a material!
existing here at this weblog, thanks admin of this web page.