Many materials may be added to water to depress both the hydrate and freezing temperatures. For many practical reasons, a thermodynamic hydrate inhibitor such as methanol or one of the glycols is injected, usually monoethylene glycol (MEG or EG). Solubility loss of MEG in the gas phase is negligible and loss to the liquid hydrocarbon phase is very low. However, methanol losses are more significant, particularly vapor phase losses. The methanol content of vapor and liquid hydrocarbon phases depend on temperature, pressure and composition. Based on the GPA-Midstream RR 149 [1] the methanol content of the gas phase can be as high as 0.075 mole % (750 PPMV) and in the liquid hydrocarbon phase as high as 0.6 mole %. Depending on the solubility losses, chemical makeup requirements for methanol can be very large and expensive for both once-through systems and methanol recovery units.
The significant amount of methanol lost to the hydrocarbon phases may cause problems for refineries, petrochemical, LNG and gas plants downstream. In gas plants where there is propane recovery the methanol will follow the propane product and can be a potential cause for propane to go off specification. Methanol has also been known to cause premature failure in molecular sieves. In refineries the methanol must be washed out of the crude/condensate, where it presents a problem in wastewater treatment. In petrochemical plants methanol is also considered poison for certain catalysts. The readers can find more detail in reference [2].
The October 2010 tip of the month (TOTM) considered the presence of methanol in the produced oil/water/gas stream and determined the quantitative traces of methanol ending up in the TEG dehydrated gas [3]. The July 2016 TOTM considered the presence of methanol in the sour gas of a sweetening unit and determined the quantitative traces of methanol ending up in the sweet gas, flash gas and acid gas streams. That tip concluded that the methydeithanolamine (MDEA) sweetening process removes a considerable amount of methanol from feed sour gas. Moreover, if the methanol content of the sour gas is high, the sweetened gas may still retain high methanol content and can cause operational troubles in the downstream processes. A modified MDEA sweetening process with 100% purging of the reflux stream can reduce the sweet gas methanol content in the range of 92% to 95% [4].
Similar to the July 2016 TOTM, this tip will consider the presence of methanol in the sour NGL stream and determine the quantitative traces of methanol ending up in the sweet NGL, flash gas and acid gas streams. To achieve this, the tip simulates a simplified MDEA gas sweetening unit by computer [5, 6]. This tip also studies the effect of feed sour NGL methanol content, and the rate of replacing condensed reflux with fresh water on the sweet NGL methanol content. For the feed sour NGL temperature of 26.7 °C (80 °F) the tip studies five inlet NGL methanol contents of 50, 250, 500, 1000, and 1500 PPM on mole basis (30, 149, 298, 596, 894 PPMw, weight basis). In each case the tip varies the rate of fresh water replacement from 0 to 100 % by an increment of 20%. The simulated results are presented graphically.
Case Study:
For the purpose of illustration, this tip considers sweetening of a sour NGL stream using MDEA. Table 1 presents its composition, standard liquid volume rate, pressure, and temperature. This tip uses ProMax [7] simulation software with “Amine Sweetening – PR” property package to perform all of the simulations.
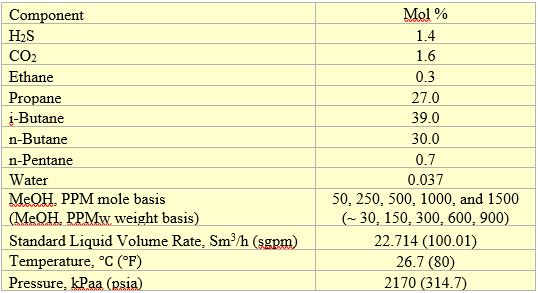
Table 1. Feed composition, volumetric flow rate and conditions
This tip used the same modified process flow diagram of Figure 1 in the July 2016 TOTM [4]. Note that the contactor is a liquid/liquid contactor rather than gas/liquid as in the July TOTM. A large fraction of methanol entering with the sour NGL leaves the sweetening unit via the treated NGL, flash gas and acid gas streams. However, some of the methanol is trapped and accumulates in the system and reaches its highest concentration in the regenerator reflux stream. In order to further lower the methanol concentration in the treated NGL, a fraction of reflux stream is purged via “Water Draw” stream and replaced with “Fresh Water.”
In Figure 1, the “Water Draw” stream removes a specified fraction of the condensed reflux (stream 10A) and the “Fresh Water” stream adds the same amount of fresh water to the process (stream 10B). To illustrate the effect of this water replacement on lowering the methanol content of the sweet gas, the fraction of condensed water removed is varied from 0 to 100% with an increment of 20% on the mole basis.
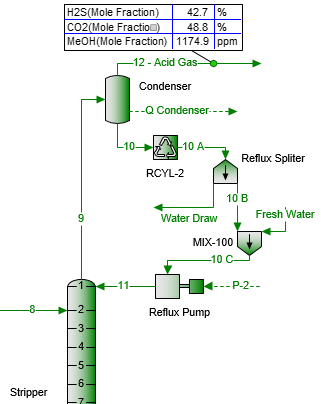
Figure 1. Schematic for replacement of a portion of reflux stream with fresh water
The following specifications/assumptions for the case study are considered:
Liquid/Liquid Contactor Column
- Feed sour NGL is saturated with water
- Number of theoretical stages = 4
- Pressure drop = 20 kPag (3 psi)
- Lean amine solution temperature = Sour NGL feed temperature 26.7 (80 )
Regenerator Column
- Number of theoretical stages = 10 (excluding condenser and reboiler)
- Rich solution feed temperature = 98.9 (210 )
- Rich solution feed pressure = 414 kPa (60 psig)
- Condenser temperature = 48.9 (120 )
- Pressure drop = 28 kPa (4 psi)
- Bottom pressure = 110 kPag (16 psig)Heat Exchangers
Reboiler duty = 132 kg of steam/m3 of amine solution (1.1 lbm/gallon) times amine circulation rate
- Lean amine cooler pressure drop = 35 kPa (5 psi)
- Rich side pressure = 35 kPa (5 psi)
- Lean side pressure = 35 kPa (5 psi)
Pump
- Discharge Pressure = Feed sour NGL pressure + 35 kPa (5 psi)
- Efficiency = 65 %
Lean Amine Concentration and Circulation Rate
- MDEA concentration in lean amine and water solution = 50 weight %
- Standard lean amine circulation rate = 11.36 Sm3/h (50 sgpm)
This rate resulted in a total acid gas loading of ~0.003 and ~ 0.15 mole acid gases/mole of amine in the lean amine and rich amine solutions, respectively. The corresponding H2S and CO2 loadings in the lean amine solution were 0.0022 and 0.0008 mole acid gas per mole amine, respectively.
Rich Amine Solution Expansion Valve
- Flash tank pressure = 448 kPag (65 psig)
Results and Discussions:
Based on the description and specifications presented in the previous section, ProMax [7] is used to simulate the NGL treating process. For each simulation run, the following properties are reported:
Methanol concentration in:
- Sweet NGL (PPMw)
- Flash gas from the amine flash tank (PPMV)
- Acid gas from regenerator (PPMV)
Methanol concentration (wt %) in:
- Lean amine
- Condensed reflux (stream 10)
- Returned reflux (stream 11)
H2S and CO2 concentration in the sweet NGL.
The calculated H2S and CO2 concentrations in the sweet NGL were little changed by reflux methanol concentration. They were less than 1.5 and 0.2 PPMV for H2S and CO2, respectively. The presence of methanol slightly increases the H2S content of sweet NGL.
To observe the impact of circulation rate on the level of NGL sweetening, three lean MDEA circulation rates of 5.68, 11.36, and 22.71 Sm3/h (25, 50, and 100 sgpm) were considered. The variation of sweet NGL methanol content as a function of the molar % of the reflux stream purged with fresh water is presented in Figures 2 through 4 for the sweet NGL, flash gas, and acid gas streams, respectively. These figures are for 11.36 Sm3/h (50 sgpm) lean MDEA rate. Similar diagrams were generated for the other two lean MDEA circulation rates.
Figure 2 presents the variation of the methanol content in the sweet NGL stream as a function of the reflux rate replacement with fresh water for five methanol contents (PPMw, weight basis) in the feed sour NGL. Note the y-axis is logarithmic.
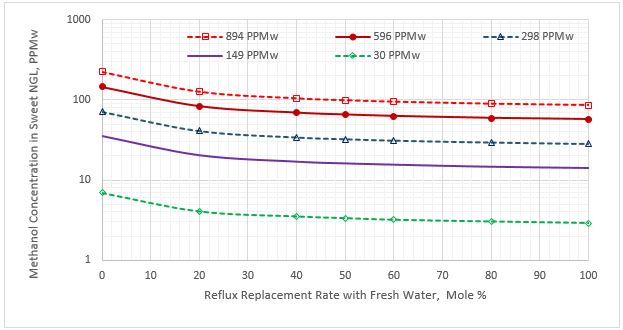
Figure 2. Methanol content in the sweet NGL stream vs reflux rate replacement for five sour NGL methanol concentrations
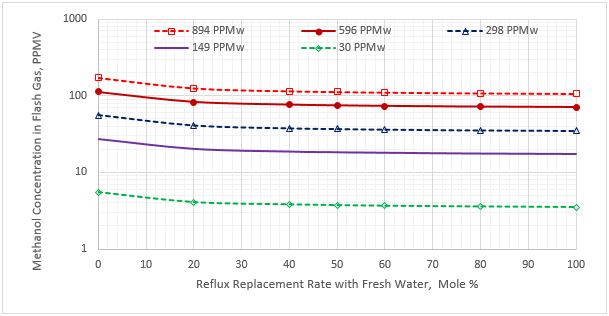
Figure 3. Methanol content in the flash gas stream vs reflux rate replacement for five sour NGL methanol concentrations
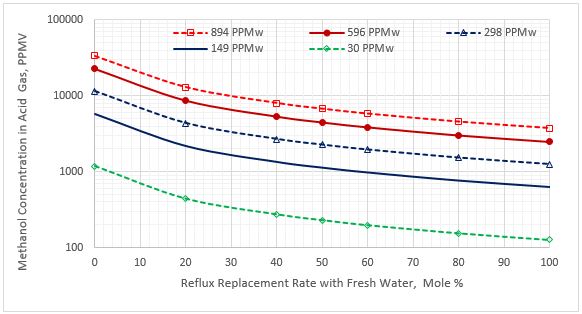
Figure 4. Methanol content in the acid gas stream vs reflux rate replacement for five sour NGL methanol concentrations
Figure 2 indicates that as the percentage of purging the reflux stream with fresh water increases, the methanol content of sweet NGL decreases. Figure 3 presents a similar trend for methanol content in the flash gas stream. Figure 4 presents the variation of the methanol content in the acid gas stream as a function of the reflux rate replacement with fresh water.
To show the impact of the lean MDEA circulation rate quantitatively, the percent of methanol content reduction for several streams was calculated by the following equations for five sour NGL methanol contents.

or
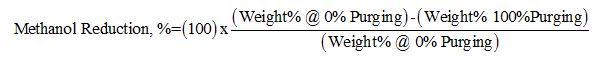
Figures 2 through 4 and the calculation results indicate that the effect of sour NGL methanol contents on the percent of methanol reduction in different streams is small. For example, the sweet NGL methanol content reduction varied within 1.5 % (i.e. 59.9% to 61.4% for sour NGL methanol contents of 30 and 894 PPMw, respectively); therefore, Table 2 presents only the average percent reduction of the five sour NGL methanol contents for each lean MDEA circulation rate. Table 2 indicates that as the lean MDEA circulation rate increases, more methanol concentration reduction takes place in the listed streams. Note that the sweet NGL methanol content reductions are the same as the reductions in the lean amine stream. This is expected because the lean amine and sweet NGL streams are almost in equilibrium with each other.
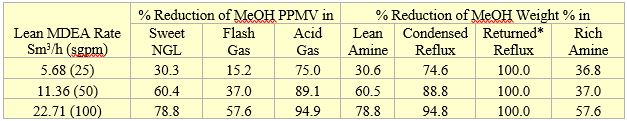
Table 2. Average percent methanol content reduction in different streams for three lean MDEA circulation rates (*Fresh water free of methanol)
The variation of methanol content as a function of the molar % of the reflux stream replaced with the fresh water is presented in Figures 5 through 7 for the lean amine, purged reflux, and replaced reflux streams, respectively. These figures are for 11.36 Sm3/h (50 sgpm) lean MDEA rate. Similar diagrams were generated for the other two lean MDEA circulation rates.
Figure 5 indicates that as the percent of purging the reflux stream with the fresh water increases, the methanol content of the lean amine decreases. Figure 6 presents a similar trend for methanol content in the purge reflux stream. Figure 7 presents a different trend for methanol content in the returned reflux stream. For 100% purging, the reduction of methanol content is 100% for the five sour NGL methanol contents.
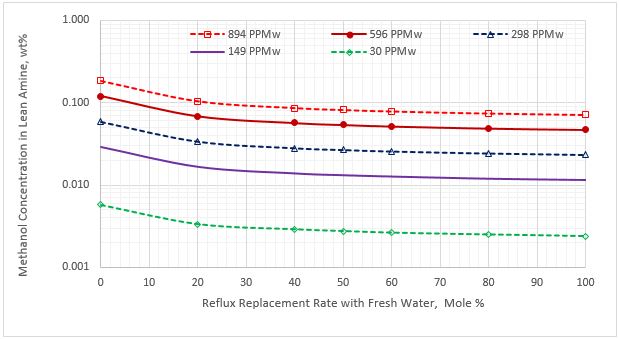
Figure 5. Methanol content in the lean amine stream vs reflux rate replacement for five sour NGL methanol concentrations
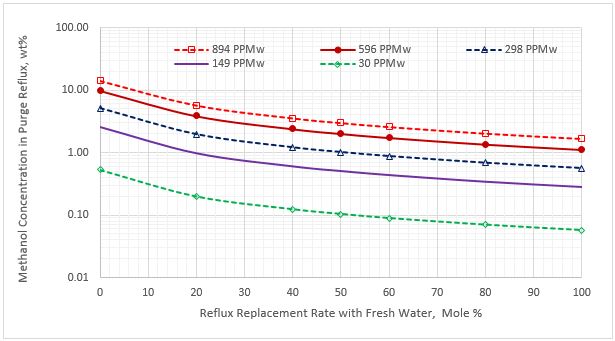
Figure 6. Methanol content in the purge reflux stream vs reflux rate replacement for five sour NGL methanol concentrations
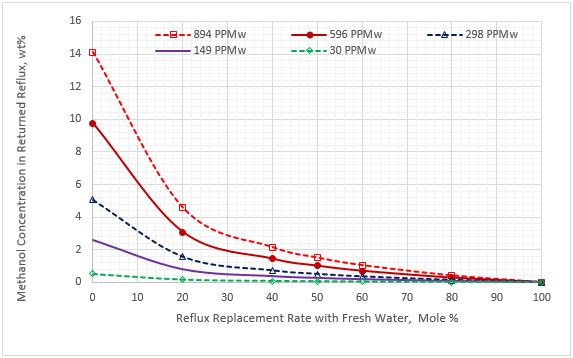
Figure 7. Methanol content in the replaced reflux stream vs reflux rate replacement for five sour NGL methanol concentrations
Figures 8A and 8B present the methanol concentration profiles in the liquid streams leaving the stages in the regenerator column. These profiles are for lean MDEA circulation rate of 11.36 Sm3/h (50 sgpm), 0 and 100 % purge of reflux, and sour NGL methanol content of 30 and 894 PPMw. These figures indicate that the maximum methanol concentration occurs in the reflux stream with 0 % purging. With 100 % purging, the reflux stream contains no methanol. Similar profiles were observed for the two lower and higher lean MDEA circulation rates.
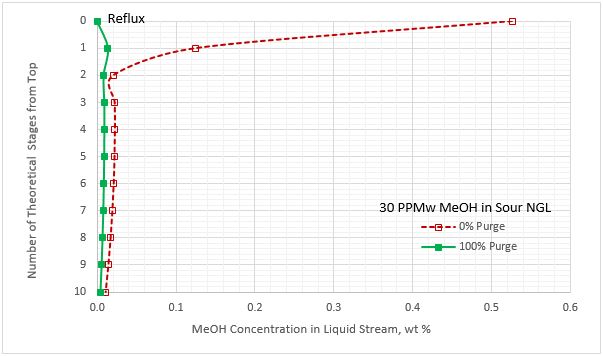
Figure 8A. Methanol content profile for liquid stream leaving the stages in the regenerator
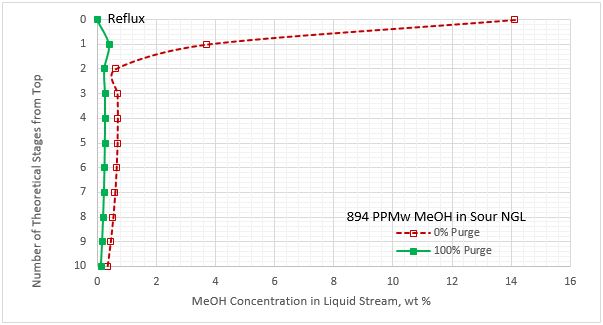
Figure 8B. Methanol content profile for liquid stream leaving the stages in the regenerator
Conclusions:
Based on the results obtained for the considered case study, this TOTM presents the following conclusions:
- Similar to the gas sweetening process, the MDEA liquid sweetening process removes a considerable amount of methanol from feed sour NGL. Moreover, if the methanol content of the sour NGL is high, the sweetened NGL may still retain high methanol content and can cause operational troubles in the downstream processes.
- The highest concentration of methanol content due to entrapment of methanol in the system is in the condensed reflux stream 10 of Figure 1 (see also Figure 8).
- Provisions of purging reflux (Water Draw) and its replacement with “Fresh Water” (Figure 1) can improve methanol recovery.
- The effect of sour NGL methanol content on the percent of methanol reduction in different streams is small.
- The basic MDEA sweetening process reduced the sweet NGL methanol content (PPMV) by ~60%, ~75% and ~80% for the lean MDEA circulation rate of 5.68, 11.36, and 22.71 Sm3/h (25, 50, and 100 sgpm), respectively.
- The modified MDEA sweetening process with 100% purging of the reflux stream reduced the sweet NGL methanol content (PPMV) by ~75%, ~90% and ~95% for the lean MDEA circulation rates of 5.68, 11.36, 22.71 Sm3/h (25, 50, and 100 sgpm), respectively.
- The purged reflux stream, which contains methanol should be disposed of properly or treated within the plant. The treated recovered water may be reused as fresh water in the sweetening process.
- In NGL sweetening, the residual amine in the NGL can also be a problem. The treated NGL usually goes through a water wash step. This step would also remove MeOH in the treated NGL stream. The wash water could be used as make-up in the regenerator purge loop. Water wash of the NGL would have a major impact on NGL quality, but only minor impact of flash gas and acid gas.
Methanol is both a Hazardous Air Pollutant (HAP) and a Volatile Organic Compound (VOC). It is regulated by the US EPA under the Clean Air Act. Operators therefore need to make sure that it is disposed of properly when purged. However, they must also consider its release into the atmosphere. This means that if there is no sulfur present, the acid gas likely cannot be vented without exceeding HAP/VOC thresholds. It must be sent to a control device, and even there, depending on the size of the plant, the operator may still bump into threshold limits.
To learn more about similar cases and how to minimize operational troubles, we suggest attending our G6 (Gas Treating and Sulfur Recovery), G4 (Gas Conditioning and Processing), G5 (Advanced Applications in Gas Processing), and PF4 (Oil Production and Processing Facilities) courses.
PetroSkills | Campbell offers consulting expertise on this subject and many others. For more information about these services, visit our website at http://petroskills.com/consulting, or email us at consulting@PetroSkills.com.
By: Dr. Mahmood Moshfeghian
References:
- Gas Processors Association, “GPA RR-149: Vapor-Liquid and Vapor-Liquid-Liquid Methanol or Ethylene Glycol Solutions,” 1995. Equilibrium for H2S, CO2, Selected Light Hydrocarbons and a Gas Condensate in Aqueous
- O’Brien, D., Mejorada, J., Addington, L., “Adjusting Gas Treatment Strategies to Resolve Methanol Issues,” Proceedings of Lawrence Reid Gas Conditioning Conference, Norman, Oklahoma, 2016.
- Moshfeghian, M., October 2010 tip of the month, PetroSkills | John M. Campbell, 2010.
- Moshfeghian, M., July 2016 tip of the month, PetroSkills | John M. Campbell, 2016.
- Maddox, R.N., and Morgan, D.J., Gas Conditioning and Processing, Volume 4: Gas treating and sulfur Recovery, Campbell Petroleum Series, Norman, Oklahoma, 1998.
- Campbell, J.M., Gas Conditioning and Processing, Volume 2: The Equipment Modules, 9th Edition, 1st Printing, Editors Hubbard, R. and Snow –McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, 2014.
- ProMax 4.0, Bryan Research and Engineering, Inc., Bryan, Texas, 2016.

Thanks for any other magnificent post. The place else could anyone get that kind
of information in such a perfect manner of writing? I have a presentation next week, and I am on the search
for such information.