OSHA mentions “near-misses” as recordable requirements in several passages as: “An unplanned and unforeseeable event that could have resulted, but did not result, in human injury, damage to property or the environment or other form of loss”. And we know that all industrial maintenance organizations have a history of reactive, run-to-failure-then-run-to fix, maintenance management behaviors. JMC’s emphasis on process and equipment reliability and operations management helps to bring facilities out of the reactive mode, but reactive maintenance jobs are still all too prevalent. Some, or many of these reactive jobs “could have resulted, but did not result, in human injury, damage to property or the environment or other form of loss”.
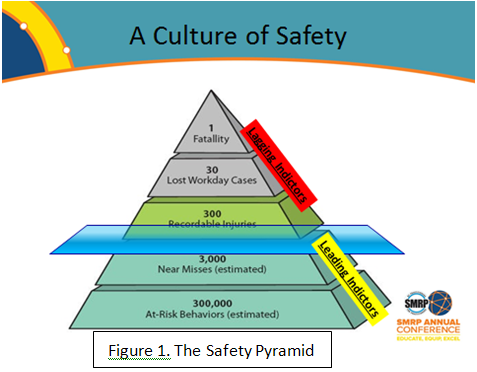
Safety is everyone’s number one goal. Most corporate safety programs define near-misses, but few connect the dots between recordable incidents and the degree of reactive, unplanned maintenance work. The famous safety pyramid is quite familiar, but that’s only the tip of the safety iceberg. Below the ‘water line’ of recordable first aids (lagging indicators) lie near-misses and, at the base of it all, safe behavior. These are the leading indicators of our safety performance. This Tip of the Month will tie reactive maintenance and safe behavior together.
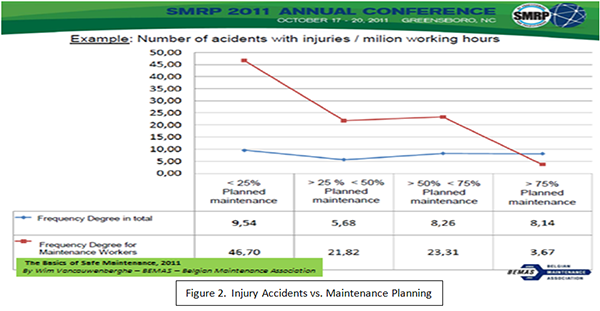
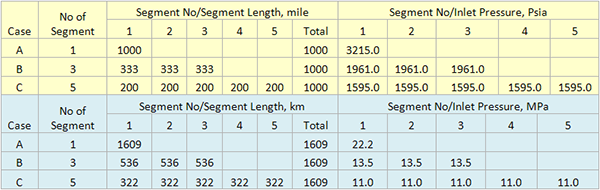
Recent data, compiled by Belgian’s BEMAS, clearly links accidents with injuries to the percent of reactive maintenance work (the opposite of planned and scheduled work). If, indeed, a company uses the near-miss definition, how can it not require the recording of some, if not all, unplanned maintenance jobs?
An iceberg is a good metaphor for Safety; most of its mass lies beneath the surface and we see only the tip. The safety pyramid compares the quantity of accidents in layers with fatality on the top and reportable incidents on the bottom. But the real basis of safe behavior lies underneath the reporting surface and is comprised of near misses and unsafe behaviors.
For over 33 years I have been focused on driving down unplanned maintenance jobs through training and consulting on control of work. We all should know that planned maintenance is simply safer! But we have been reluctant to tie urgent, reactive jobs to unsafe practices. In 2012, it’s time to ask “Should unplanned maintenance jobs be recorded as near-misses”?
In a nearly parallel development path, our emphasis and understanding of safe work environments has also been refined. With the help of several catastrophic events, like the Texas
City refinery and, more recently Deepwater Horizons, and many smaller injury-causing accidents, our industry has put safety on the front burner.
A useful way to look at the safety pyramid (Figure 1) is to draw the dividing plane at what is reported and not reported. This brings behavior-based safety programs, which we all talk about, into perspective. A key point here is the separation of leading indicators and lagging indicators. It’s obvious to record Incidents and Accidents after they happen, but less obvious to capture near-misses and instill safe behaviors.
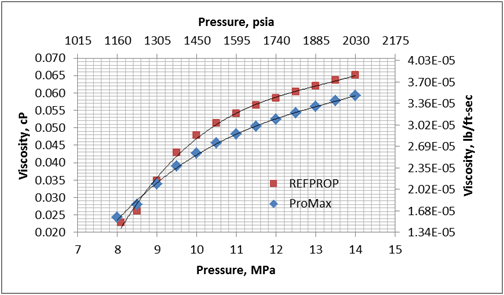
Recent data (Figure 2), presented by Wim Vancauwenberghe [1] of the Belgian Maintenance Association (BEMAS) at last year’s SMRP (Society for Maintenance and Reliability Professionals) annual conference shows the impact of unplanned maintenance jobs on the rate of accidents with injuries; and subsequent reduction in injuries as the percentage of planned work increases.
This raises the question in this paper, and it’s time we asked.
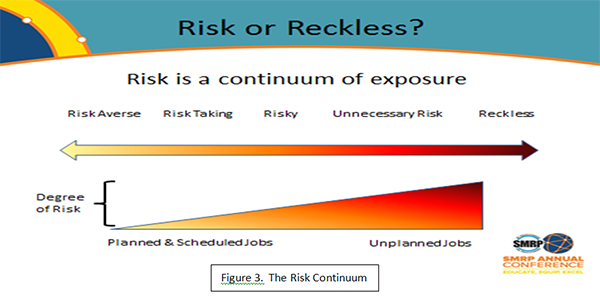
Clearly, not every unplanned maintenance job involves the same level of risk. We can use a risk-based approach as in Figure 3 to indicate when an unplanned job becomes a near miss. When we look at the spectrum of behavior from risk averse upwards to reckless, we can begin to establish some range of criteria for defining what to report. Applying the risk spectrum to the nature of unplanned jobs, we would expect risk to increase due to some factors. Typical risk matrices compare event likelihood to its consequence to determine level of risk. Should we develop something similar for unplanned jobs? This tip attempts to describe the conditions that would determine the level of risk in jobs. Perhaps there are companies who have successfully addressed this issue and, hopefully they will contribute to this discussion.

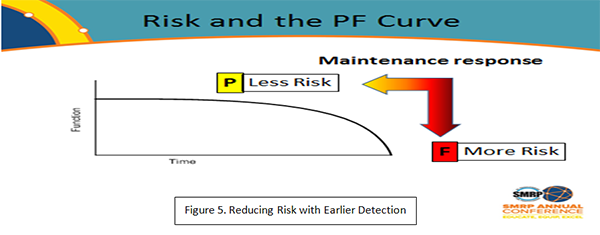
OSHA distinguishes Accident, Incident and Near Miss with the definitions in Figure 4. However, trying to define what ‘could’ have happened in every urgent job opens a Pandora’s Box that probably wouldn’t be very productive. On the other hand, we could approach the near miss issue by defining ‘failure’ more carefully. Taking the familiar P-F curve, we might be able to say that earlier definition of failure at the P point and the subsequent maintenance job would inherently be safer than reacting to a failure at the F point. Figure 5 shows how that might work. We could say that anytime we have an unexpected complete failure of equipment, it must be reported as a near miss, whereas, if we detect a potential failure and plan and schedule the maintenance action before complete functional failure, it wouldn’t need to be reported because it is not a near miss.
If we are going to require near misses to be reported, another issue is raised: How is a near miss to be reported? What do we do with the report? How much information/data is required on such a report? If we’re going to require a report, we will have to define what and how much detail is required.
There are several possible uses for a near miss report. Whatever decision we take, will impact our staff with more information gathering tasks. What is it worth? How can we successfully use the report to lower near misses and effect safer behavior? Or, drive down reactive maintenance work?
- Used as a way to ‘speak up’ with the rest of the crew and raise their awareness would not require as much information about what happened,
- Determine preventability of the near miss with root cause analysis would require a great deal more information.
The fundamental questions are:
- How do we raise the awareness of near misses with the target to reduce them?
- What distinguishes a near miss from an incident?
- If unplanned maintenance jobs carry higher safety risk, how do we break our reactive maintenance habits?
- What criteria do we use to define levels of risk?
In order to determine how the professional SMRP audience would distinguish the reportability of near misses, several situations were presented for the participants to vote using the following choices:
- Do it and report as a near miss
- Near miss, Speak up!
- Risky behavior, don’t tell anyone
- No risk, just do it!
- Do not proceed without a planned work order
The sample situations were:
- Urgent restart of a 100 hp motor after unexpected stoppage
- Talking on your cell phone while driving
- Vehicle crossing your path while running a yellow light
- 5 lb. (2.27 kg) hammer dropped from scaffolding
- Hurrying to replace hydraulic fitting without lock out, tag out
- 2 ton lifting sling frayed, but go ahead and use it
Results of this voting may be published in a subsequent TOTM, or send an email to the author, perry.lovelace@jmcampbell.com.
In conclusion, we have raised the question and some of the issues around the question “Should unplanned maintenance jobs be recorded as near misses?” There is not a simple answer and our profession must continue to explore the issues and make efforts to create a safer workplace through planned and scheduled maintenance work.
To this end, JMC offers training related to reducing unexpected failures:
- The Operations Management discipline is directly focused on reduction of unplanned events through better control of work,
- Operator Training broadens facilities operators’ competencies by teaching how facilities work and why certain events happen,
- Mechanical and Reliability disciplines help identify onset of equipment failures. Reliable equipment is safer equipment,
- Many facilities use contractors for maintenance; their safety is also important. JMC’s Supply Chain and Procurement disciplines concentrate on better contractor relationships in our SC-41 course.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G40 (Process/Facility Fundamentals), G4 (Gas Conditioning and Processing), G5 (Gas Conditioning and Processing-Special), P81 (CO2 Surface Facilities), PF4 (Oil Production and Processing Facilities), and PL 4 (Fundamentals of Onshore and Offshore Pipeline Systems) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Perry Lovelace, Sr. Staff Instructor
References:
- Vancauwenberghe, Wim; The Basics of Safe Maintenance; The Belgian Maintenance Association; 2011.



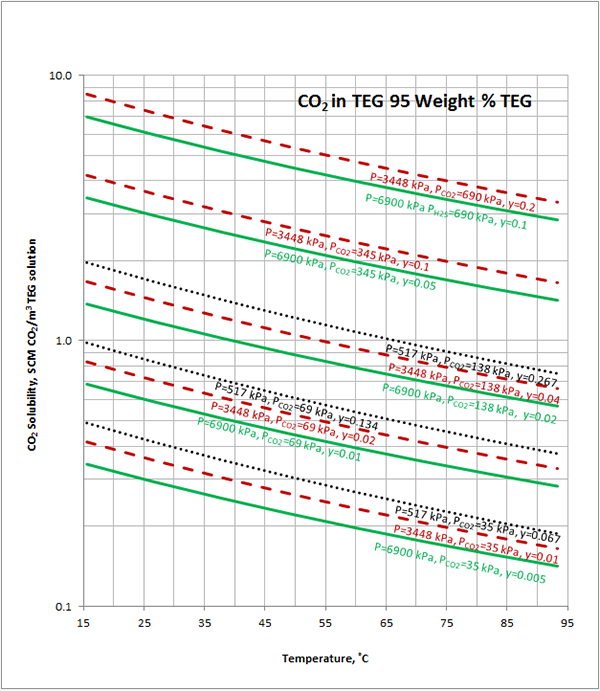
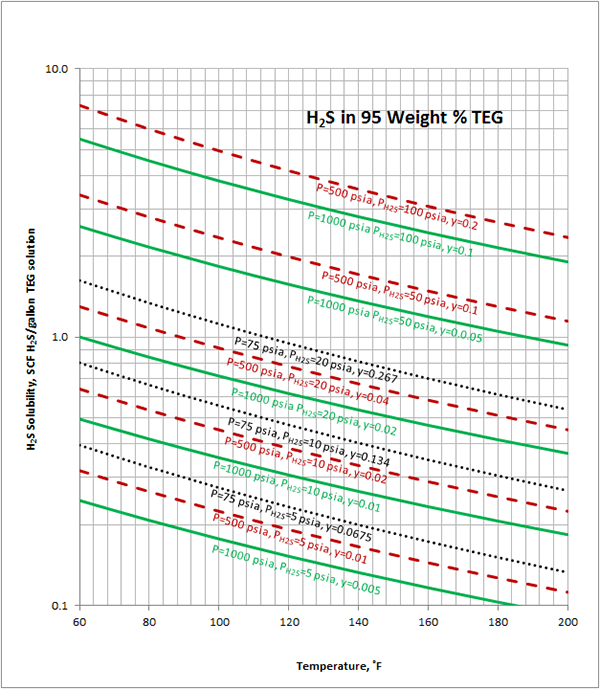
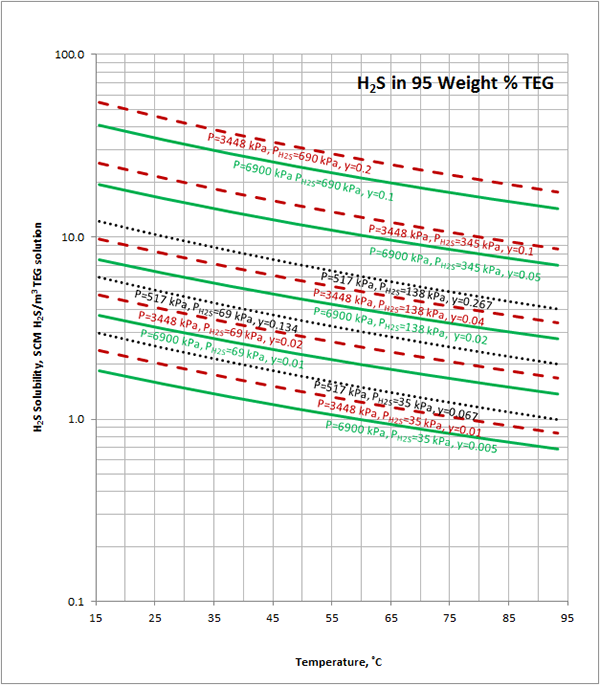
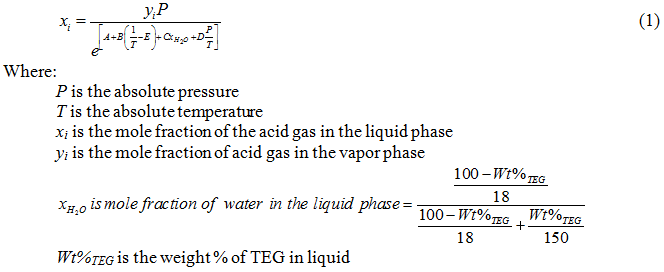




Excelent.
For the most part, I agree with the statements in this month’s tip. Not only at my current worklace have I witnessed “crash maintenance” become the standard. In my opinion this is not only hazardous because of the potential that the machine in question could come apart while an operator is in the immediate area. Once the unit has gone past its scheduled preventative maintenance period, it also fails much more often, potentially causing a lot of overtime, thus burning out the staff, due to after hours callouts. Should the unit in question be a critical piece of machinery, it could have a domino effect upon failure, shutting down the rest, or a large portion of the plant as well.
Perry has hit the nail on the head here handsomely.
An urgent restart of the motor sounds to me like the operators did not find out why it went down in the first place and just hit the starter – not following practise.
Talking on my cell phone while driving is ILLEGAL IN ALBERTA – you can be charged and convicted – let’s never go there.
Oh a vehicle crossed my path while I am running a yellow light – How do I explain that to my wife and/or children as a safety professional? I approach intersections with lights as if the light will change at the worst possible time.
The last three incidents are near misses.
Learn from the mistakes of others – you will not live long enough to make them all yourself.
Thanks so much with regard to giving me personally an update on this subject matter on your web site. Please know that if a fresh post becomes available or in the event that any modifications occur about the current write-up, I would be considering reading more and focusing on how to make good use of those approaches you talk about. Thanks for your time and consideration of people by making this blog available.
I must thank you for the efforts you’ve put in penning this
website. I am hoping to check out the same
high-grade blog posts by you in the future as well.
In truth, your creative writing abilities has encouraged me to get my own, personal blog now 😉
Thank you, I have recently been searching for info approximately this topic for
a while and yours is the greatest I’ve found out so far.
But, what concerning the bottom line? Are you sure about the supply?
What’s Taking place i am new to this, I stumbled upon this I have discovered It positively helpful and it has aided me out loads.
I am hoping to contribute & aid other customers like its aided me.
Great job.
The Best aircon servicing singapore
Thank you, I’ve recently been hunting for information about this subject matter for ages and yours is the best I have found so far.
DreamProxies.com — cheapest professional private proxies with 50% discount! Top notch quality, Limitless proxies, Super speed along with Least expensive rates — simply $0.25 for each proxy! Very best personal proxies only on DreamProxies.com
DreamProxies.com : lowest priced professional private proxies with 50% discounted! Top notch quality, Unlimited proxies, Excellent speed and also Least expensive price ranges : only $0.25 for every proxy! Ideal personal proxies only on DreamProxies.com